Introduction:
The technique known as sodium dodecyl sulphate-polyacrylamide gel electrophoresis, also known as SDS-PAGE, is most frequently used in genetic, biotechnology, biochemistry, and molecular biology laboratories to separate proteins from a mixed sample and to identify and quantify a single protein therein.
The relative molecular weight (Mr) of denatured polypeptide chains and the purity of a protein preparation can both be determined quickly and accurately using this method.
In SDS-PAGE, the sample that will be placed to the gel is first treated with the anionic detergent SDS, which denatures the sample’s proteins and binds closely to the protein molecules. In proportion to its length, the SDS molecules provide the polypeptide with a relatively equal negative charge. All proteins move through the gel matrix in the direction of the anode when an electric current is introduced across the gel.
Principle of SDS-PAGE:
The principle of SDS-PAGE states that when an electric field is applied, a charged molecule will go to the electrode with the opposite sign. The relative mobility of charged species affects how the charged molecules are separated from one another.
Due to decreased resistance during electrophoresis, the smaller molecules migrate more quickly. The rate of migration is also influenced by the proteins’ charge and structure. The influence of the proteins’ structure and charge is eliminated using sodium dodecyl sulphate and polyacrylamide, and the proteins are separated as per length of the polypeptide chain.
The sample buffer for SDS-PAGE contains SDS, an anionic detergent. Together with some reducing chemicals such as 2-Mercaptoethanol (BME) or Dithiothreitol (DTT), SDS tends to disrupt the tertiary structure of proteins by rupturing their disulfide bridges and creating a negative charge. Based on the length of their polypeptide chains, the protein is ultimately separated electrophoretically.
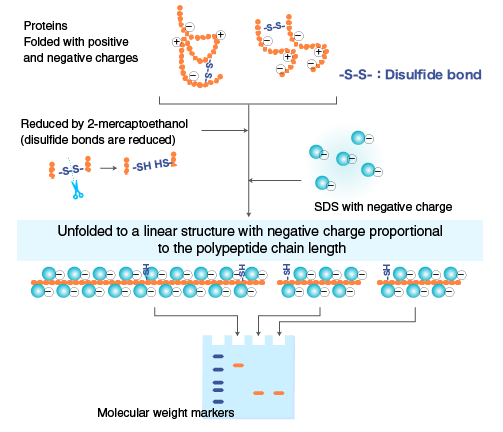
Fig: Action of SDS on Protein
The SDS-coated proteins have a uniform charge: mass ratio, permitting SDS-PAGE to distinguish proteins according to size in this manner. Because of the sieve effect of the gel matrix, proteins with smaller masses move through the gel more quickly than proteins with larger masses.
Using a polyacrylamide gel matrix, the main goal of SDS-PAGE is to electrophoretically separate particular proteins from a variety of materials based on their size. The reaction between acrylamide and N, N’ –methylene-bis-acrylamide produces polymerized acrylamide (polyacrylamide), a matrix that resembles gel and is appropriate for the separation of proteins. By altering the cross-concentration, linking’s one can control the gel’s hardness and softness. The gel will get firmer with increased cross-linking, which will slow down molecule migration. In contrast, molecule migration is accelerated with loose gel.
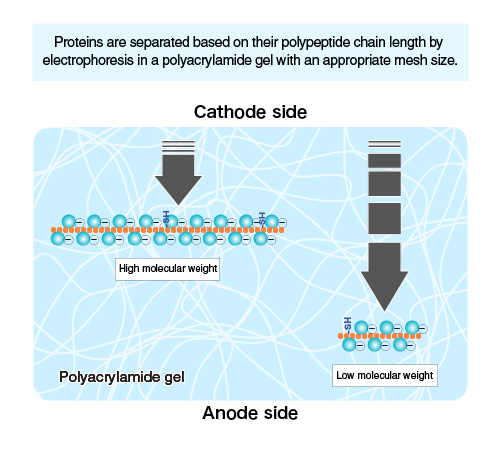
Fig: Separation of protein on the base of molecular weight
Components/Requirements:
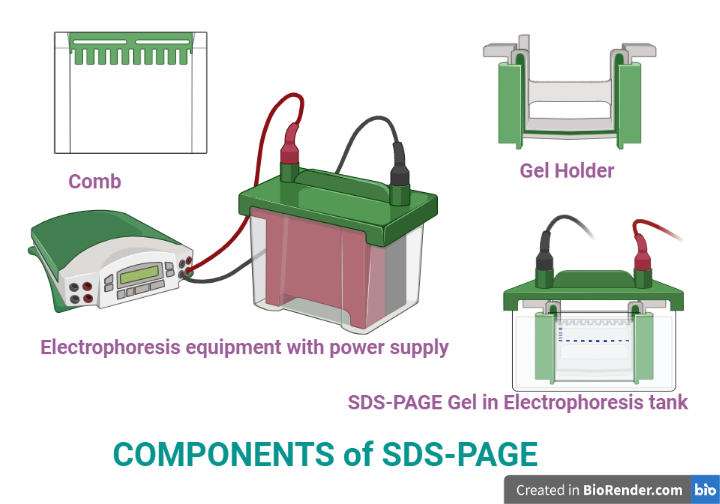
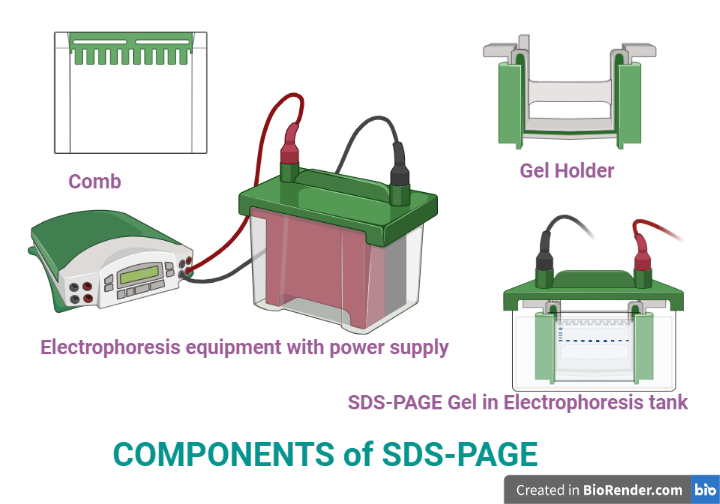
Fig: Components of SDS-PAGE
Power Supplies
It is used to convert the AC current to DC current in order to supply power to the electrophoresis chamber. The electrodes and anode maintained the potential difference in the chamber.
Gels
Basically, there are two types of gel Separating or resolving gel and stacking or spacer gel.
Electrophoresis Chambers
Protein Samples
Running Buffer
Ions in the running buffer allow current to flow through the gel. When proteins are placed into wells at the top edge and current is supplied, the proteins are pulled by the current through the matrix slab and separated in the gel.
Staining and Destaining Buffer
SDS-PAGE gels are often stained with Coomassie blue stain.
Protein Ladder
It is also known as protein standards or protein ladders that is employed to determine the molecular weight of target proteins and to track the development of electrophoretic separation.
Buffers for Protein Electrophoresis
- Protein Gel-loading dye for SDS-PAGE
- TG Tris-Glycine
- TGS Tris-Glycine-SDS
- SDS Sodium Dodecyl Sulfate
- PBS Phosphate Buffered Saline
- TBS Tris-Buffered Saline
Detergents/Denaturing Agents
- SDS 20% solution
- Triton x-100
- Tween 20
- Tween 80
- N-octl-b-d-glucpyranoside
Acrylamide, Bis-Acrylamide, and Catalysts
- Acrylamide Solution, 40%
- Bis-Acrylamide Solution, 2%
- Ammonium Persulfate (APS)
- Sodium Persulfate
- TEMED (Tetramethylethylenediamine)
Protease Inhibitors
- Benzamidine.Hcl
- Leupeptin hemisulfate
- Pepstatin A
Separating or resolving gel
Stacking or spacer gel
Protein ladder
Procedure and methodology of SDS-PAGE:
Sample preparation
The protein is diluted using SDS-PAGE sample buffer and heat at 100°C for 3 minutes. In order to prevent any tertiary protein folding, a reducing agent such dithiothreitol (DTT) or 2-mercaptoethanol (BME) is also added to weaken the disulfide bonds.
While 2-mercaptoethanol releases sulfhydryl groups, which allow polypeptide chains with an excess negative charge similar to mass ratio to be released, SDS binds to the protein tightly and creates a negative charge. Then the tubes are centrifuged at 15,000 rpm for 1 minute at 4°C and the separated supernatant is used further in SDS – PAGE processes.
Preparation of polyacrylamide gel
A buffer solution, acrylamide, N, N’-methylene-bis-acrylamide (BIS), and other components are combined to prepare an electrophoretic gel.
Ammonium persulfate, a free radical source, and a stabilizer are added during the polymerization of the gel, and the mixture is degassed or butanol is added to stop the production of bubbles. The glass plate’s spaces are filled with a comb, which is then given time to polymerize.
There are essentially two types of gel: the resolving layer and the stacking layer. The bottom (resolving) layer contains more acrylamide and has a higher pH (8.8), whereas the top (stacking) layer has less acrylamide and a lower pH (6.8). SDS-PAGE is run in a discontinuous buffer system (two different buffers are used). After the polymerisation of the gel, it will be places in the gel cassettes.
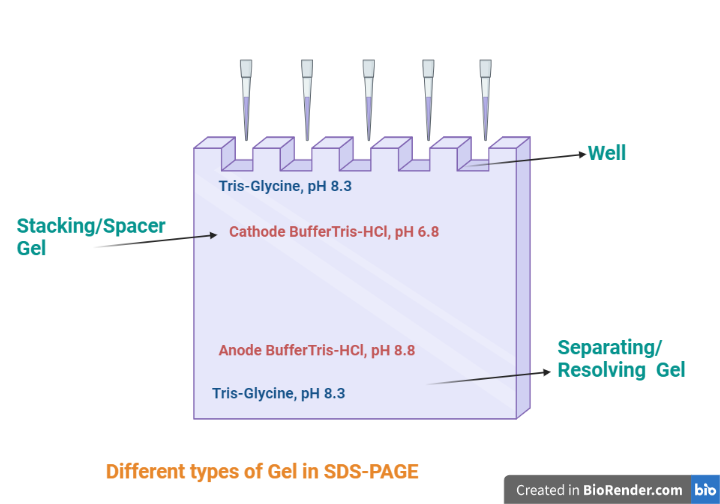
Fig: Different types of gel in SDS-PAGE
Electrophoresis
The loading buffer is mixed with around 30 ml of the denatured sample and added to the well before being allowed to run at 30 mA for an hour. Protein molecules that are negatively charged move through the gel in the direction of an electrode that is positively charged as an electric current is applied.
According to their molecular size, each protein molecule migrates at a variable velocity; a small-sized molecule moves more quickly than a large-sized one. The pace of migration is also accelerated by high voltage. However, it might denature the gel’s structure.
It may take an hour or more to completely separate protein molecules, after which they are labelled and viewed.
Staining and visualization
Coomassie Brilliant Blue (CBB) is commonly used for the detection of proteins in sodium dodecyl sulphate-polyacrylamide gel electrophoresis. CBB in addition to methanol, acetic acid, and water is know as staining solution which are mixed in the ration (3:1:4) which not only stain the protein but also fix the protein in place.
Additionally, the entire gel will be stained, making it impossible to see the protein bands. However, the dye may then be selectively removed from the protein-free regions of the gel using a comparable solvent in which the dye is absent. This solution is known as destaining which is composed of methanol, acetic acid and water. The gel is dyed, leaving only the blue-stained protein bands visible, which can help with result analysis and interpretation.
For rapid sample quantification in a gel, this general protein visualization is used.
The most recent technology includes the use of antibodies attached with a dye or enzyme that identifies the specific structure of proteins in order to visualize specific proteins.
By transferring proteins from a gel to a membrane that may be probed with antibodies, Western blotting is also connected to protein visualization.
In this way, through comparing the distance travelled by the unknown molecules and the marker, it is possible to estimate the molecular mass of an unknown protein sample.
Applications:
- Since the migration rate of a protein coated with SDS is inversely proportional to the logarithm of its molecular weight (MW), SDS-PAGE is a reliable method for determining the molecular weight of an unknown protein.
- Used in peptide mapping, size and number of polypeptide subunits and comparative study in the polypeptide composition of different structures.
- It is employed to determine the purity of the proteins.
- In clinical settings, it is used in HIV test to separate the HIV proteins.
- To analyses post-translational modifications.
- Characterization of purified vaccine by SDS-PAGE and Western blotting analysis.
References:
- Hames, B.D. (1990). One-dimensional Polyacrylamide Gel Electrophoresis. In Gel Electrophoresis of Proteins, A Practical Approach, 2nd Edition, (ed. B.D. Hames and D. Rickwood), Oxford University Press, New York.
- Shapiro AL, Viñuela E, & Maizel JV, Jr. (1967) Molecular weight estimation of polypeptide chains
- by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun 28: 815–820.
- Steinberg TH (2009). Protein gel staining methods: An introduction and overview. Method Enzymol 463: 542-563.