Introduction:
The term “blotting” refers to the process of transferring biological samples from a gel to a membrane and then detecting them on the membrane’s surface.
Towbin, et al. introduced the western blot experiment, or western blotting (also known as immunoblotting since an antibody is employed to precisely detect its antigen), in 1979, and it is now a standard protein analysis technique.
The process is named Western blotting because it is similar to Southern blotting. Southern blotting is used to detect DNA, while Western blotting is used to detect proteins.
Because of the specificity of the antibody-antigen interaction, a target protein can be found in a complex protein mixture. Western blotting can provide both qualitative and semi-quantitative information about a protein.
Principle:
The technique consists of three major processes:
- Electrophoresis: The first step in a western blotting procedure is to separate the macromolecules in a sample using gel electrophoresis.
- Blotting: Subsequently, the separated molecules are transferred or blotted onto a second matrix, generally a nitrocellulose or polyvinylidene difluoride (PVDF) membrane.
Detection: Marking target protein using a proper primary and secondary antibody or chromogenic substrates or chemiluminescent substrate to visualize.
Steps:
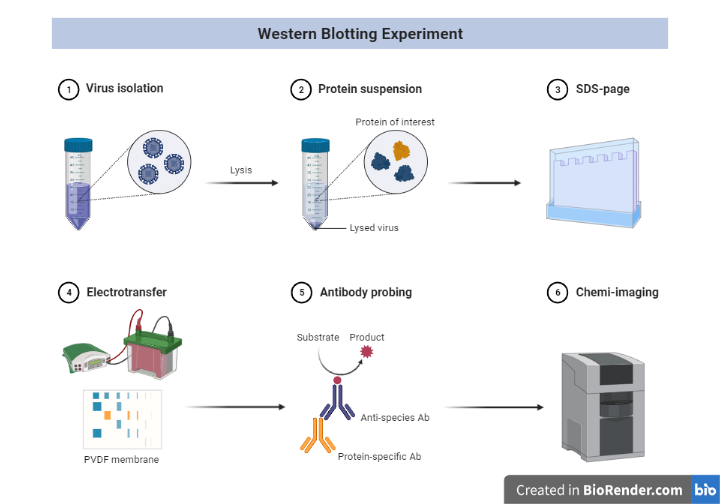
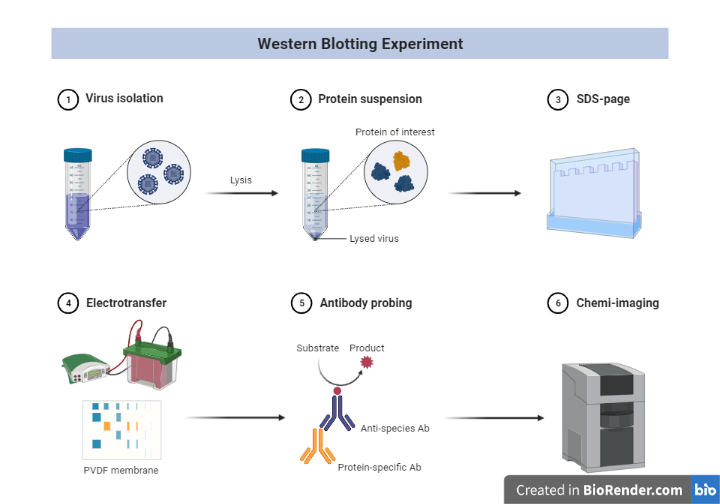
Fig: Schematic representation of western blotting
Sample Preparation and extraction of protein
For efficient separation of protein bands in electrophoresis and western blot detection, appropriate sample preparation is essential. Because protein samples are so diverse, no single sample preparation method or buffer will work for all of them. At 2-8 °C, all processes for extracting protein from cells or tissue (fresh or frozen) must be completed. Cells and tissues must be lysed to release the proteins of interest before they can be run on a gel. A handy, ready-to-use reagent for soluble protein extraction that is versatile and easy to use is readily available and can be used to achieve the project’s goals and objectives.
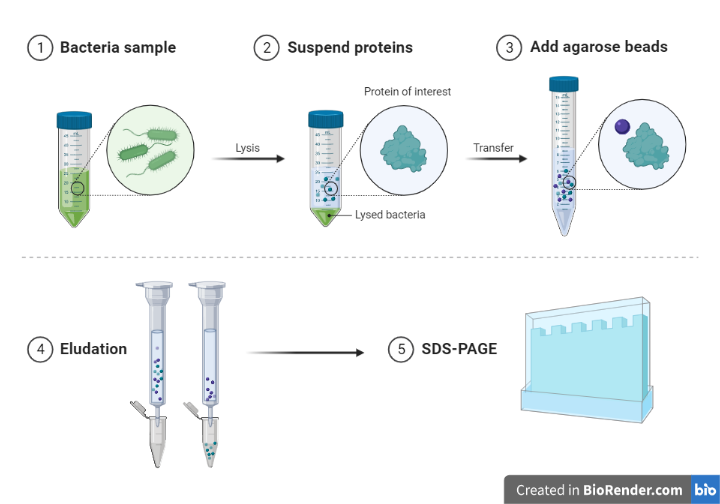
Fig: Protein sample preparation
Electrophoretic separation of proteins
Gel electrophoresis is a process in which charged molecules, such as protein or DNA, are separated by physical properties as they move through a gel under the influence of an electrical current. To identify individual proteins in a complicated sample or evaluate many proteins within a single sample, polyacrylamide gel electrophoresis (PAGE) is often used. PAGE, when used in conjunction with western blotting, is a powerful analytical method for determining the mass, charge, purity, or existence of a protein.
Transferring proteins to a membrane
After the protein mixture has been separated, the resultant proteins must be transferred to a membrane in order for antibodies to probe them.
The protein must then be transferred from the gel to a membrane following electrophoresis. Diffusion transfer, capillary transfer, vacuum blotting transfer, and electroelution are just a few of the techniques that have been employed to perform.
Because of its speed and efficiency, electroelution or electrophoretic transfer is the most commonly used method for protein transfer. To transfer proteins from the gel to the membrane, this approach relies on its electrophoretic mobility.
Electrophoretic transfer of proteins is achieved by sandwiching a protein-containing polyacrylamide gel between two electrodes submerged in a conducting solution (Transfer solution/buffers).
Porous pads and filter paper are used in the process to help with the transfer. Proteins migrate out of the polyacrylamide gel and onto the membrane’s surface when an electric field is applied, where they become firmly bound.
Fig: Transfer of protein in blotting
What is the membrane used for?
Basically, two types of membranes are used in Western blotting applications that include nitrocellulose and polyvinylidene fluoride (PVDF). Pre-cut, pre-assembled western blot membrane/filter paper sandwiches perfectly fit in the gel make protein transfer easier and more convenient.
Membranes made of PVDF (polyvinylidene difluoride) and nitrocellulose are available in a variety of pore diameters and dimensions to accommodate the needs of various applications.
Because the features of a protein (such as charge, hydrophobicity, etc) influence its abilities to attach to membrane surfaces, determining the best membrane may necessitate screening specific proteins on several membranes.
Nitrocellulose membrane
To ensure high-quality transfer, the nitrocellulose membrane (pore size 0.2 m) is made entirely of 100% pure nitrocellulose. Staining, immunodetection, fluorescence, and radiolabelling are all approaches that can be employed with the membrane. Due to hydrophobic and electrostatic interactions, the proteins bind to the membrane. 209 g/cm2 is the protein binding capability.
PVDF membranes
The PVDF membrane has a better binding capacity than nitrocellulose (0.2 m pore size, low fluorescence). Without any pre-treatment with alcohols, the PVDF membrane is preactivated and ready to use. Staining, immunodetection, fluorescence, and radiolabelling are among ways that can be used to detect the membrane. Hydrophobic interactions cause the proteins to attach to the membrane. The binding capability of proteins is 240 g/cm2.
Blocking the Membrane
Next, the membrane is blocked to prevent any nonspecific binding of antibodies to the surface of the membrane.
Western blot blocking is a very important step of western blotting, as it prevents antibodies from binding to the membrane non-specifically. Blocking is often made with BSA or non-fat dried milk diluted in TBST/PBST buffers.
Note: 1X Tris-Buffered Saline, 0.1% Tween 20 Detergent (TBST)
1X Phosphate-Buffered Saline, 0.1% Tween 20 Detergent (PBST)
Generally, it is advised to incubate for 1 hour or more in membrane blocking solution at 4°C under agitation. Followed by rinsing the membrane for 5 seconds in TBST after the incubation of blocking.
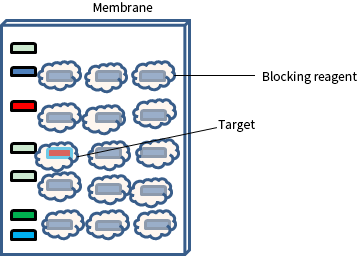
Fig: Membrane blocking for Western Blot
Detection
The detection step of a western blot experiment has a wide range of procedures.
Direct versus indirect detection is a typical distinction. An enzyme- or fluorophore-conjugated primary antibody is utilized to detect the antigen of interest on the blot in the direct detection approach. Most researchers prefer the indirect detection method for a variety of reasons; hence this direct method is not extensively employed.
An unlabelled primary antibody is utilized to attach to the antigen in the indirect detection approach. After that, an enzyme- or fluorophore-conjugated secondary antibody is used to detect the primary antibody. Biotin, fluorescent probes, and enzyme conjugates such as horseradish peroxidase (HRP) or alkaline phosphatase are examples of labels (or conjugated compounds) (AP). In comparison to the direct method, the indirect method has numerous advantages.
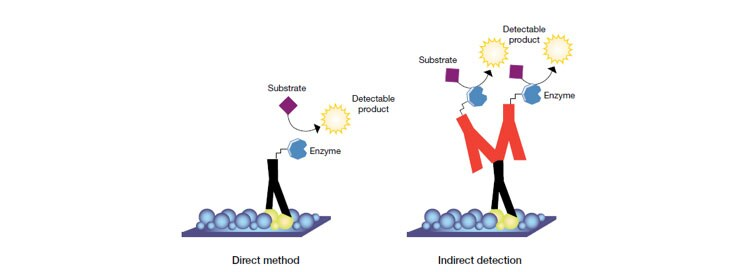
Fig: Direct and indirect method of detection in western blotting
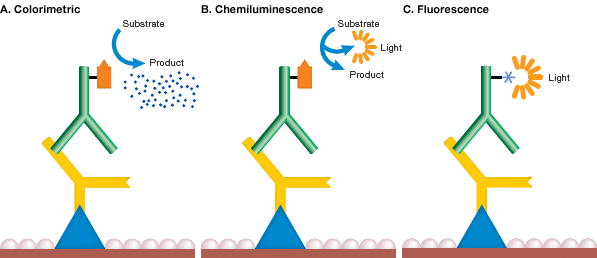
Fig: Method of detection in western blotting
While there are many different tags that can be attached to a secondary or primary antibody, the detection method employed in a western blotting experiment will limit the types of tags that can be utilized.
Radioisotopes were once widely utilized, but they are expensive, have a short shelf life and require specific handling and disposal. Enzymes and fluorophores are other possible labels.
While X-ray film can provide semi-quantitative data, digital imaging is more sensitive due to the wide dynamic range of detection, allowing researchers to collect quantitative data from western blots.
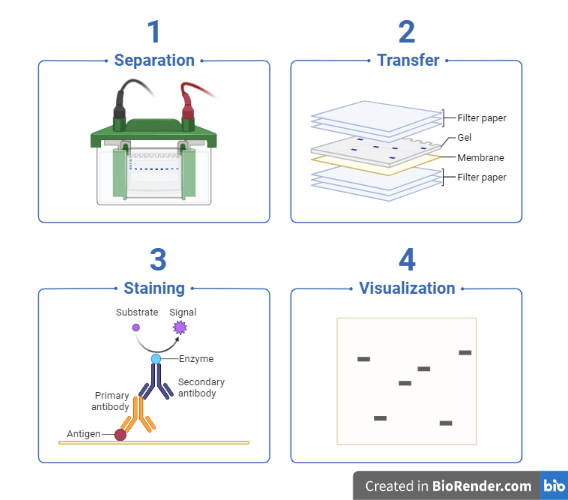
Fig: Work flow of western blotting
Applications:
In biochemistry, Western blotting is widely used to identify the presence of specific proteins, to determine the extent of post-translational modifications, to verify protein expression in cloning applications, to analyse protein and biomarker expression levels, to map antibody epitopes, and to test for disease markers in clinical settings.
- Detecting or Characterizing Protein Expression
- Specific antibodies in the serum can be used to diagnose neurocysticercosis and tubercular meningitis.
- Identification of a specific protein from a heterogenous mixture of proteins in protein biology
- Demonstrating Antibody Specificity
- Diagnosis
References:
- Wu L, Hu X, Tang H, Han Z, Chen Y. Valid application of western blotting. Mol Biol Rep. 2014 May;41(5):3517-20. doi: 10.1007/s11033-014-3215-5. Epub 2014 Feb 8. PMID: 24510387
- Towbin, et al. (1979) Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets: Procedure and Some Applications. PNAS 76:4350–4354.
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350-4. PMID: 388439
- Blevins T. Northern blotting techniques for small RNAs. Methods Mol Biol. 2010;631:87-107. doi: 10.1007/978-1-60761-646-7_9. PMID: 20204871.
- Kurien, B.T. and Scofield, R.H. (2009) Introduction to Protein Blotting. In: Protein blotting and detection: methods and protocols. New York: Humana Press. pp 9–22.