Introduction:
- A carbohydrate is an organic compound that is consists only of carbon, hydrogen and oxygen. Carbohydrates make up the bulk of organic substances on earth and perform numerous roles in living things.
- Several qualitative tests have been devised to detect members of this biologically significant class of compounds. These tests will utilize a test reagent that will yield a colour change after reacting with specific functional groups of the compounds being tested.
Test 1. Molisch Test for Carbohydrates:
The Molisch test is a general test for the presence of carbohydrates. The Molisch Reagent is a 95% ethanol solution of alpha-naphthol.
Principle:
The dehydration reaction is used to detect the presence of carbohydrate in Molisch’s test. When concentrated H2SO4 is added to the sample, the carbohydrates dehydrate into aldehyde.
Furfural (produced by dehydration of pentoses or pentosans) or hydroxymethylfurfural is the aldehyde formed (produced by the dehydration of hexoses or hexosans). The -naphthol in the molisch reagent then reacts with the aldehyde to form a purple condensation product.
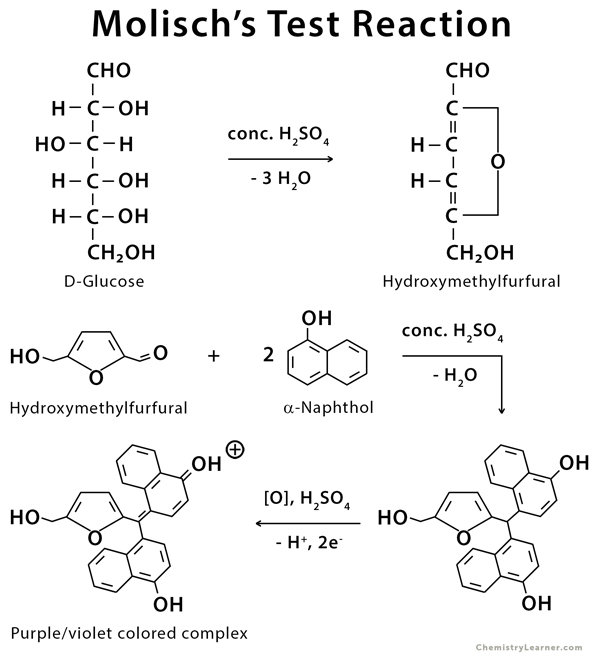
Fig: Prinicple of Molisch Test
Procedure:
- In a test tube, place 2 mL of a known carbohydrate solution and 1 drop of Molisch’s reagent (10% -naphthol in ethanol).
- Pour 1-2 mL of concentrated H2SO4 down the side of the test tube, forming a layer at the bottom of the tube.
- A purple colour at the interface of the sugar and acid indicates a positive test.
- Examine the colour at the interface of two layers and compare your results to a control test.
Result interpretation:
Positive result: Violet ring appears at the junction of two layers, i.e., between the sugar and acid.
Negative result: Green or brown colour appears.
Application: It detects the presence of all carbohydrates.
Test 2. Anthrone Test for Carbohydrates:
This is a carbohydrate analysis test. It is a quick and easy way to quantify carbohydrates that are either free or bound to lipids or proteins.
Principle:
Anthrone testing is a general test for all carbohydrates. Carbohydrate is dehydrated in this test as well when it reacts with concentrated H2SO4 to form furfural. This furfural reacts with anthrone to form a bluish green complex.
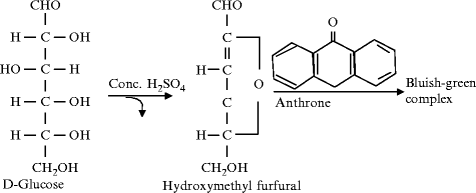
Fig: Principle of Anthrome test
Procedure:
- Place 1ml of sample in a test tube
- In another tube, place 1ml of distilled water as a control.
- Add 2ml of anthrone reagent to each tube.
- All of the tube’s contents should be thoroughly mixed.
- Keep an eye out for a bluish green colour change.
Result interpretation:
Positive result: bluish green appears
Negative result: No green or brown colour appears.
Application: It detects the presence of all carbohydrates.
Test 3. Test for Reducing Sugars:
A reducing sugar is any sugar that contains an aldehyde or ketone group in solution. Sugar enolization under alkaline conditions is an important consideration in reduction tests. The ability of a sugar to reduce alkaline test reagents is dependent on the availability of an aldehyde or keto group for reduction reactions.
A number of sugars, particularly disaccharides and polysaccharides, have glycosidic linkages, which involve bonding a carbohydrate (sugar) molecule to another, and thus there is no reducing group on the sugar; for example, sucrose, glycogen, starch, and dextrin. In the case of reducing sugars, the presence of alkali causes extensive enolization, especially at high pH and temperature. This increases the susceptibility to oxidation reactions compared to neutral or acidic pH. As a result, these sugars can reduce Cu+2 to Cu+, Ag+ to Ag, and so on.
Fehling’s, Benedict’s, and Barfoed’s tests are the most commonly used for detecting reducing sugars.
Benedict’s Test
Copper alkaline solutions are reduced by sugars containing a free aldehyde or ketone group, resulting in the formation of coloured cuprous oxide. Benedict’s solution is made up of copper sulphate, sodium carbonate, and sodium citrate (pH 10.5)
Principle: By reacting with an alkaline reagent, such as Benedict’s solution, the reducing sugar will be reduced into enediols. Depending on the concentration of the reducing sugar, the precipitate ranges from green to brick red. The colour change is caused by the reduction reaction of copper (II) to copper (I) in the solution, which results in a red precipitate.
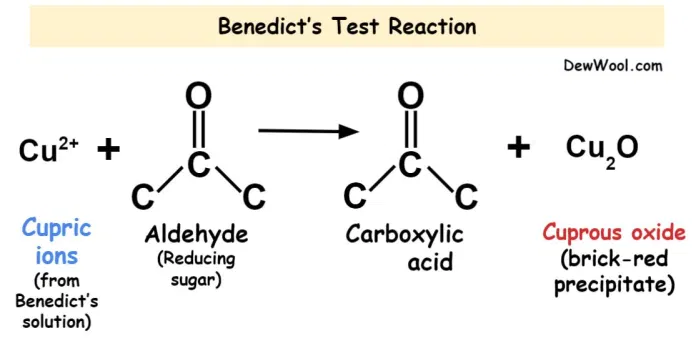
Fig: Principle of Benedict’s Test
Procedure:
- 5 mL of Benedict’s solution should be mixed with 1 mL of the test solution before shaking the tubes.
- The tube should be heated for 3 minutes in a boiling water bath and allowed it to cool immediately.
- A positive test for reducing sugars is the appearance of a green, red, or yellow precipitate.
Positive result: Green to brick-red precipitate forms.
Negative result: Colour remains unchanged.
Application: It detects the presence of reducing carbohydrates.
Barfoed’s Test
In the presence of disaccharides, this reaction will detect reducing monosaccharides. This reagent detects reducing sugars in an acidic solution by using copper ions. Copper acetate in dilute acetic acid is Barfoed’s reagent (pH 4.6).
Principle:
In Barfoed’s test, the copper ion in the solution oxidizes the reducing monosaccharide to form a carboxylic acid and copper (I) oxide, resulting in the formation of a red coloured precipitate.
Procedure:
- 1 mL of the solution to be tested + 3 mL of freshly prepared Barfoed’s reagent
- Place test tubes in a boiling water bath for 3 minutes.
- Remove the tubes from the bath and set aside to cool. The formation of a green, red, or yellow precipitate indicates that monosaccharides have been reduced.
- Appearance of the red colour after 3-4 minutes of heating will indicated the presence of disaccharides in the given test sample.
Result interpretation:
Positive result: Green to brick-red precipitate forms.
Negative result: Colour remains unchanged.
Fehling’s Test
This method is also useful for analysing reducing sugars. As a chemical reagent, it employs Fehling’s solutions A and B. Fehling’s solution A contains copper sulphate pentahydrate in 1 L of distilled water. Fehling’s solution B contains potassium sodium tartrate and sodium hydroxide in 1 litre of distilled water.
Principle:
In the Fehling’s test, a reduction reaction occurs between the reducing sugar’s aldehyde or keto groups and the alkaline cupric hydroxide, which later reduces to cuprous oxide. In solution, this cuprous oxide produces a brick red precipitate.
Procedure:
- In a test tube, place 2 ml of the test sample.
- Then, in equal parts, add Fehling’s A and B solutions to the above sample.
- After that, immerse the test tube in a hot water bath for 3 minutes and observe the colour change.
Result interpretation:
Positive result: Brick-red precipitate forms.
Negative result: Colour remains unchanged.
Application: It detects the presence of reducing carbohydrates.
Picric Acid Test
Picric acid test is one of the tests for the detection of reducing sugars.
Principle:
The picric acid test for carbohydrates is a highly sensitive chemical test for the presence of reducing sugars. Picric Acid (toxic yellow crystalline solid), also known chemically as 2,4,6-trinitrophenol (TNP), is formed when the reducing sugars react with Picric Acid. All monosaccharides and disaccharides with a potentially free aldehyde or ketone group have reducing properties. Sugars with a free aldehyde or ketone group have a reducing property. When placed in an alkaline solution, they reduce some organic acids. To make the solution alkaline or basic, Sodium Carbonate (Na2CO3) is added. Thus, reducing sugars convert picric acid (yellow solution) to picramic acid (mahogany red solution).
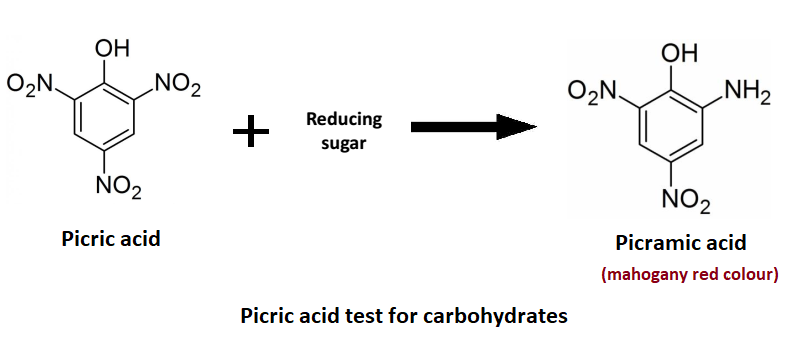
Fig: Principle of picric acid test
Reagents:
Saturated picric acid: Dissolve 13 g Picric Acid in 100mL distilled water, boil and cool.
Sodium Carbonate (10% solution).
Procedure:
- 1mL saturated picric acid to 1mL test solution, followed by 0.5mL 10% soda carbonate solution
- In a boiling water bath, heat the test tube.
- Look at the color of the solution.
Result interpretation:
The presence of reducing sugar in the solution is indicated by the presence of red colour.
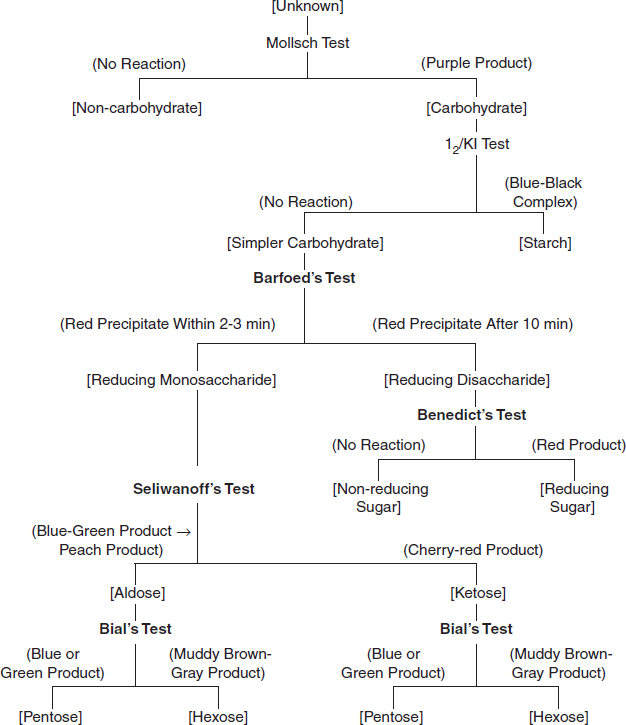
Fig: Flow chart of classifying an unknown carbohydrate
Test 4. Bial’s Test for Pentoses:
It is most frequently used to detect pentose sugars or pentoses. We can also use color change to distinguish pentoses from hexoses using this method. The Bial’s test employs Bial’s solution, which contains orcinol, hydrochloric acid, and ferric chloride.
Principle:
The pentoses react with the Bial’s reagent, converting it into furfural derivatives via HCl dehydration. Then, in the presence of ferric ions, orcinol and furfural condense and form a green compound.
Procedure:
- 2 mL of the solution to be tested + 5 mL of Bial’s reagent
- Gently bring the tube to a boil. Allow the tube to cool.
- The formation of a green solution or precipitate indicates a positive reaction.
Test 5. Iodine Test for Starch and Glycogen:
It is widely used to detect starch in solutions. Iodine acts as an “Indicator” in an iodine test.
Principle: In the Iodine test, an iodine solution reacts with the starch (which contains -amylose and amylopectin polymers). The reaction between starch and iodine produces a starch-iodine complex, which gives the solution a blue-black colour.
Procedure:
- Add 2-3 drops of Gram iodine solution to 5 mL of the solution to be tested.
- Starch produces a blue-black colour.
- A positive glycogen test produces a brown-blue colour. A negative test is distinguished by the test reagent’s brown-yellow colour.
Result interpretation:
Positive result: Gives blue-black colour to the solution.
Negative result: Gives a brown-yellow colour to the solution.
Application: It detects polysaccharides like starch.
Test 6. Seliwanoff’s Test:
It is most commonly used to detect ketosis or keto-sugars. We can also use color change to distinguish Ketoses from Aldoses using this method. Seliwanoff’s test employs a reagent containing 0.5 g of resorcinol per litre of 10% HCl.
Principle:
In the Seliwanoff’s test, ketoses react with the HCl in the Seliwanoff’s reagent to produce furfural derivatives due to dehydration. The resorcinol and furfural then react to give the solution a deep red color.
Procedure:
- Add 1 ml of the test solution in the test tube.
- Then pour in 3 mL of Seliwanoff’s reagent.
- For 1 minute, place the solution in a water bath.
- Allow to cool and examine the solution to the color change.
Result interpretation:
Positive result: Gives a deep red colour to the solution.
Negative result: Colour remains unchanged.
Application of Carbohydrate Tests:
- Food Industry
- Clinical Diagnostics
- Agriculture and Plant Sciences
- Pharmaceutical Industry
- Biochemical Research
- Fermentation Industries
- Environmental Analysis
References:
- The A, Saccharides A. Experiment 1- Qualitative Analysis of Carbohydrates Tests on Carbohydrates :
- Elzagheid, M.I., 2018. Laboratory activities to introduce carbohydrates qualitative analysis to college students. World J Chem Educ, 6(2), pp.82-86.
- Katoch, R., 2011. Carbohydrate Estimations. In Analytical Techniques in Biochemistry and Molecular Biology (pp. 67-76). Springer, New York, NY.
- http://www.chem.boun.edu.tr/
- Rathod Zalak R., et al. “A Review on Qualitative and Quantitative Analysis of Carbohydrates Extracted from Bacteria”. Acta Scientific Pharmaceutical Sciences 6.3 (2022): 20-28.
- Benedict, S.R., 1909. A reagent for the detection of reducing sugars. Journal of Biological Chemistry, 5(5), pp.485-487.
