Introduction:
Optogenetics is an elegant method of accurately regulating and monitoring the biological processes of a cell, group of cells, tissues, or organs by using optical system and genetic engineering technologies. The use of the technology known as optogenetics has completely changed the way that neuroscience is studied since it allows for the precise real-time control and manipulation of the activity of individual neurons and brain circuits.
Discovery:
The discovery of optogenetics began with the study of light-sensitive proteins in single-celled organisms such as bacteria and algae. In 1979, Francis Crick suggested the possibility of genetically modifying neurons to make them light-sensitive. However, it was not until 2002 that the first light-activated ion channel, Channelrhodopsin-2 (ChR2), was discovered in a species of green algae, Chlamydomonas reinhardtii, by Georg Nagel and colleagues.
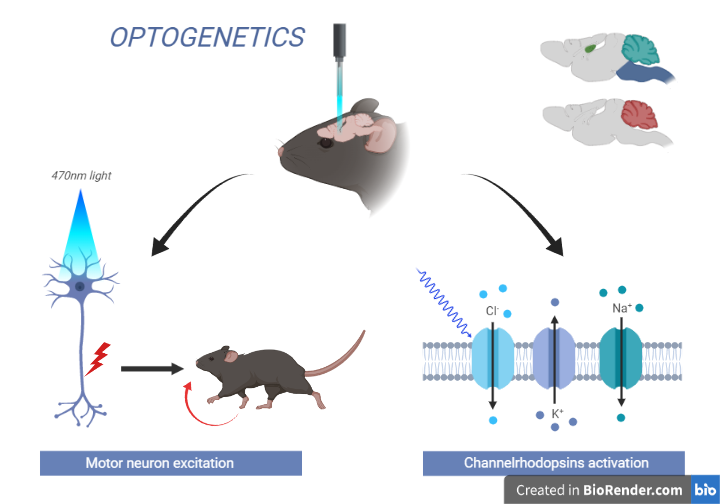
Fig: Optogenetics
In 2005, Karl Deisseroth and colleagues at Stanford University were the first to use ChR2 to control the activity of neurons in living animals. They introduced the gene encoding for ChR2 into neurons in the brains of mice, and demonstrated that shining light on the neurons caused them to fire action potentials. This groundbreaking work demonstrated the potential of optogenetics to control and manipulate neural activity in real-time with high precision.
Drosophila Optogenetic Experiment:
Drosophila Melanogaster (fruit flies) optogenetic experiments entail genetically altering the flies to express a light-sensitive protein in particular neurons or circuits of interest, followed by light stimulation via a fiber-optic cable to control neuronal activity. After that, the modification can be seen using electrophysiological or imaging methods, and it can be evaluated to determine how the neurons or circuits are involved in behavior or other physiological processes. Therefore, we can advance our understanding of the neurological underpinnings of behavior and other physiological processes by using optogenetics, which offers a potent tool for precise and focused modulation of brain activity in a well-characterized genetic model system.
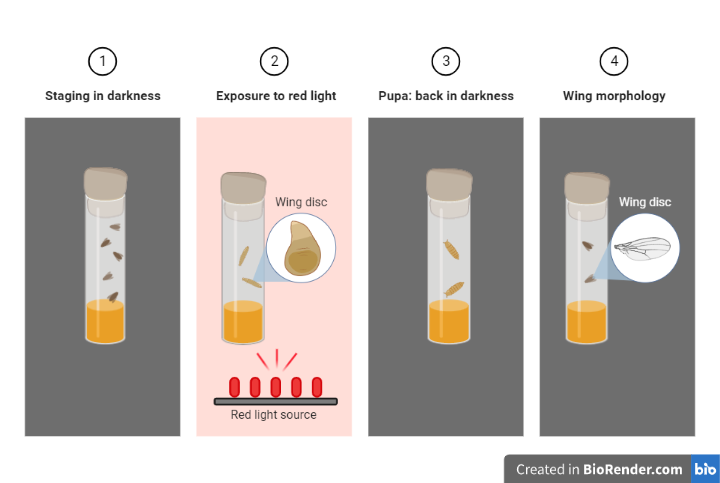
Fig: Drosophila Optogenetic Experiment
Technologies of optogenetics:
Optogenetics involves the use of several technologies to manipulate and monitor neural activity in response to light stimulation.
Light-sensitive proteins: Optogenetics relies on the use of light-sensitive proteins, such as Channelrhodopsin-2 (ChR2), that can be genetically introduced into neurons. These proteins respond to light of a specific wavelength, allowing for precise manipulation of neural activity.
Light delivery systems: Light delivery systems are used to deliver light of a specific wavelength to the neurons expressing the light-sensitive proteins. These systems can be custom-made to target specific regions of the brain or specific neurons.
Electrophysiology: Electrophysiology techniques, such as patch-clamp recording, are used to measure changes in neural activity in response to light stimulation. This allows researchers to monitor the effects of optogenetic manipulation on neural activity in real-time.
Imaging techniques: Imaging techniques, such as two-photon microscopy, can be used to visualize changes in neural activity in response to optogenetic manipulation. This allows for a deeper understanding of the underlying neural processes.
Behavioural assays: Behavioural assays can be used to assess the effects of optogenetic manipulation on animal behaviour. This allows researchers to link changes in neural activity to specific behaviours.
Optogenetic proteins in research and medicine:
| Protein | Wavelength | Response | Application |
| Channelrhodopsin-2 (ChR2) | 470 nm (blue) | Ion channel allowing depolarization | Neural activation, behaviour manipulation |
| Archaerhodopsin (Arch) | 530 nm (green) | Proton pump allowing hyperpolarization | Neural inhibition, behaviour manipulation |
| Halorhodopsin (NpHR) | 590 nm (yellow) | Chloride pump allowing hyperpolarization | Neural inhibition, behaviour manipulation |
| Step-function opsins (SFOs) | Varies | Ion channels allowing temporally precise neural activation | Temporally precise neural activation, behaviour manipulation |
| Phytochromes | 650-750 nm (red) | Light-sensitive proteins allowing neural activation | Neural activation, behaviour manipulation |
| Jaws | 470 nm (blue) | Modified ChR2 with slower off-rate | Temporally precise neural activation, behaviour manipulation |
| KillerRed | 525 nm (green) | Produces reactive oxygen species | Cell ablation, tissue damage |
Applications:
With several applications in both basic science and medicine, optogenetics is a rapidly growing area. The study of brain circuits and behaviour is one of optogenetics’ most important uses in neuroscience. Additionally, optogenetic tools can be utilized to create more specialized and efficient treatments for a variety of psychiatric and neurological conditions, including Parkinson’s disease, epilepsy, and depression. The use of light-sensitive proteins that can be introduced into retinal cells to restore vision is known as optogenetics, and it is being researched as a potential cure for vision loss. Optogenetics may also be utilized in gene therapy, synthetic biology, and even cardiology.
Challenges and new strategies:
Optogenetics is a fast-developing field with a lot of potential for both fundamental study and clinical use. It does, however, encounter substantial obstacles, such as restrictions on tissue penetration, specificity, safety, and ethical issues. New optogenetic technologies that can respond to various light wavelengths and are more targeted to particular cell types are being developed as a solution to these problems. To increase the effectiveness and security of optogenetic therapies, other delivery techniques like viral vectors and nanotechnology are also being investigated. In order to ensure that these techniques are utilized responsibly and ethically, researchers are now establishing ethical standards for optogenetics application in humans.
Future prospects of optogenetics:
In the future, optogenetics is expected to have a wide range of applications beyond neuroscience. Here are some potential future prospects of optogenetics:
Medical treatments: Optogenetics has the potential to revolutionize medicine by enabling doctors to selectively activate or inhibit specific cells in the body. For example, optogenetics could be used to treat diseases like Parkinson’s by activating specific neurons that produce dopamine.
Synthetic biology: Optogenetics could be used to create synthetic biological systems that respond to light. This could have applications in bioengineering, such as creating new metabolic pathways or designing new organisms with specific functions.
Neuroscience: Optogenetics will continue to be a valuable tool for studying the brain and behaviour. It may be used to investigate how specific neural circuits contribute to different behaviours and cognitive functions.
Agriculture: Optogenetics could be used to create plants that respond to light in specific ways, such as producing more fruit or resisting pests.
References:
Duebel J, Marazova K, Sahel JA. Optogenetics. Curr Opin Ophthalmol. 2015 May;26(3):226-32.
Gracheva E, Wang F, Matt A, Liang H, Fishman M, Zhou C. Developing Drosophila Melanogaster Models for Imaging and Optogenetic Control of Cardiac Function. J Vis Exp. 2022 Aug 25;(186):10.3791/63939.
Kohsaka H, Nose A. Optogenetics in Drosophila. Adv Exp Med Biol. 2021; 1293:309-320
