Introduction:
It is an analytical tool which is used to measure the mass-to-charge ratio (m/z) of one or more molecules present in an analyte sample. Eventually, the measurement can be applied to calculate the molecular weight of the sample to quantify known compounds, and to determine structure and chemical properties of molecules.
The preference for the term “mass spectrometry (MS)” over “mass spectroscopy” is indeed related to the absence of electromagnetic radiation in the technique. Unlike spectroscopy methods that involve the interaction of electromagnetic radiation with matter (such as infrared spectroscopy or nuclear magnetic resonance spectroscopy), mass spectrometry primarily relies on the manipulation of charged particles (ions) in electric and magnetic fields.
When mass spectrometry coupled with gas or liquid chromatographs, it allows the extension of analytical capabilities to the wide range of clinical applications.
Likewise, due to its ability to identify and quantify proteins in the analyte, it is an essential analytical tool in the field of proteomics.
Principle:
A beam of electron will bombardment into the analyte compounds, it will penetrate inside the compound and electron will be knock out and released from the analyte resulting in positively charged analyte known aa radical ion. And the signal form the respective radical ion is equivalent to the molecular weight of the compound.
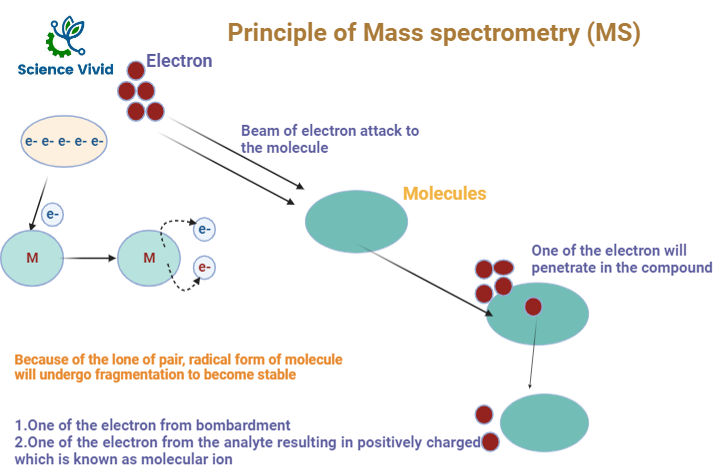
Fig: Principle of Mass Spectroscopy(MS)
Instrumentation:
Sample Injection
Sample is inserted into sample vaporization chamber. If the sample will be in the form of liquid, it is converted to vapor. If the sample is in solid, it will be dissolved into the suitable solution and sample will be further processed just liked liquid sample.
Ionization
The gas sample is immediately ionized. Before we begin, the sample is vaporized into a gas form.
Then, the sample is put into the mass spectrometer, and is immediately ionized (given a charge). This can happen by one of two methods:
Ionization can occur using Electrospray ionization (EI). Electrospray ionization is where voltage is applied, causing each particle to gain an H+ ion that will converts the sample into a gas made up of positive ions.
Ionization can also occur using electron impact ionization where an electron gun is used to fire high energy electrons at the particles. Eventually, the electrons repel a single electron out of each particle, causing them to become charged and forming 1+ ions.
Energy produced by electron beam will be approximately 70ev and ionization potential produced for the molecule is 10ev.
The ionization chamber will produce ionization potential which is minimum potential required to convert M to M+.
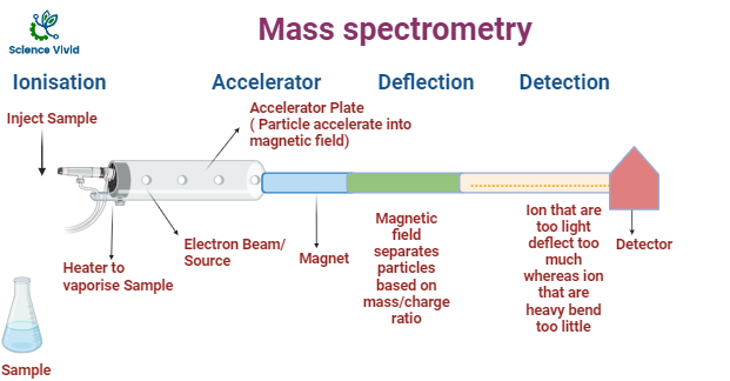
Fig: Instrumentation of Mass Spectroscopy (MS)
Electron ionisation is one of the most common form of ionisation used in GC/MS which requires a source of electrons typically at 70 eV in the form of a filament to which an electric potential is applied.
The molecules in the source are attacked with high-energy electrons, forming charged molecular ions and fragments. Molecules break down into distinct fragments based on their molecular structure. The ions created and their proportional proportions are consistent and can be used to qualitatively identify the substance.
Ion Acceleration chamber
The positively charged ions present in the sample are accelerated by an electric field and the acceleration is totally relies on the particle being charged. There is often an acceleration plate. The acceleration plate is instrumented in such a way that a negatively charged are positioned in the front to attract the ions whereas positively charged on the back to repel the ions further down the mass spectrometer. After acceleration, all ions will possess the same kinetic energy and the velocity of a particle depends on its mass and kinetic energy, so the lightest particles will move faster than the heaviest particles eventually.
Ion Drift
Next, the ions just drift through. In the next stage, the electric field is absent, so ions are not deflected and they just pass through. Lighter ions will drift faster.
Deflection
A cation will be deflected based on their mass and charge under the influence of magnetic field. Charge and deflection are directly proportional whereas mass and deflection are inversely proportional. If an ion has a greater positive charge compared to its counterparts with lesser mass it will get deflected more in the apparatus.
Detection
The ions are detected in the final stage. The ions are separated based on mass in which lighter ions are detected firstly because of the shorter drift time with respect to heavier ions.
Finally, a mass spectrum will be generated using the charged ions. The detector will use an electric field to detect the charged ions, and forms a graph known as a mass spectrum.
Applications:
Proteomics
Characterization and determination of the molecular mass of peptides, protein complexes, and sequencing of peptides, identification of posttranslational modifications, level of protein expression.
Clinical applications
Disease’s screening, development of drug, monitoring of drug therapy, used for diagnostic testing, identification of infectious agents for therapies, cancer screening and diagnosis.
Metabolomics
- Analysis of metabolic finger printing, Profiling and discovery of biomarkers and profiling of metabolic disorder.
- Pharmaceutical analysis.
- Drug discovery and absorption, analyses, metabolite screening, and preclinical development.
Environmental analysis
- Testing of drinking water testing.
- Analysis, quantification and screening of pesticide residue.
- Assessment of soil contamination assessment, monitoring of pollution, trace element and heavy metals leaching.
- Detection of food contamination.
Forensic analysis
- Identification and characterization samples from crime scenes.
- Detection of drugs, explosives, and toxic substances.
