Introduction:
HPLC (High-performance liquid chromatography) is a type of column chromatography used in biochemistry and analysis to separate, identify, and quantify active chemicals.
In the 1960s, the Liquid column chromatography system, which used low-pressure glass columns, was upgraded to the HPLC system, which used high-pressure metal columns. As a result, HPLC is essentially a more advanced version of column liquid chromatography. Instead of allowing a solvent to flow naturally through a column, it is forced through at high pressures of up to 400 atmospheres.
A column contains the stationary phase, a pump propels the mobile phase(s) through the column, and a detector displays the molecular retention times. The interaction between the stationary phase, the molecules being studied, and the solvent(s) utilized determines the retention time. The sample to be analysed is added to the mobile phase stream in a tiny quantity and is slowed down by chemical or physical interactions with the stationary phase. The amount of retardation is determined by the analyte’s nature as well as the composition of the stationary and mobile phases.
Principle:
The distribution of the analyte (sample) between a mobile phase (eluent) and a stationary phase (packing material of the column) is the basis for HPLC separation.
The liquid phase is pumped at a consistent rate into the stationary phase that is packed in the column. The analytical sample is injected into the carrier stream before being passed through the column. When the sample reaches the column, physico-chemical interactions between the analyte molecules and the stationary phase cause the sample components to be retained sequentially. According to the operating parameters, the components are eluted by the mobile phase moving at a constant rate.
After the analytes have left the column, a detection unit (such as a UV detector) detects them. A data management system (computer software) converts and records the signals, which are subsequently shown as a chromatogram. The detection and quantification of the eluted components are done using detection methods.
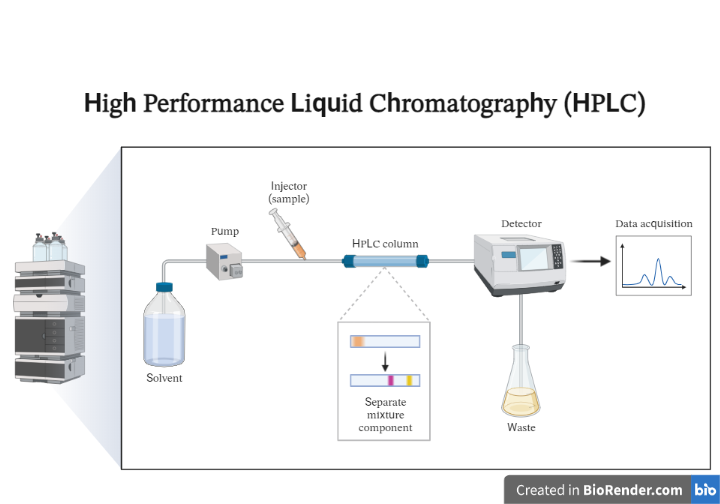
Fig: A schematic outline of HPLC
Types of HPLC:
The phase system utilized in the process determines the type of HPLC. The following HPLC types are commonly used in analysis:
Normal phase chromatography
Normal phase HPLC is a method for separating analytes based on polarity (NP-HPLC). A non-polar mobile phase and a polar stationary phase are used in NP-HPLC. The polar analyte came into contact with the polar stationary phase and is trapped eventually. The interaction between the polar analyte and the polar stationary phase increases the elution duration, and adsorption intensities increase with increased analyte polarity.
Reversed phase chromatography
The stationary phase of Reversed phase HPLC (RP-HPLC or RPC) is non-polar, and the mobile phase is aqueous and moderately polar. The theory of RPC is based on hydrophobic interactions, which are caused by repulsive forces between a polar eluent, a comparatively non-polar analyte, and a non-polar stationary phase. The contact surface area around the non-polar section of the analyte molecule after association with the ligand in the aqueous eluent is proportional to the analyte’s binding to the stationary phase.
Size exclusion chromatography
Size exclusion chromatography (SEC), also known as gel permeation chromatography or gel filtration chromatography, is a type of chromatography that primarily separates particles based on their size. It can also be used to determine the tertiary and quaternary structures of proteins and amino acids. This method is commonly used to determine the molecular weight of polysaccharides.
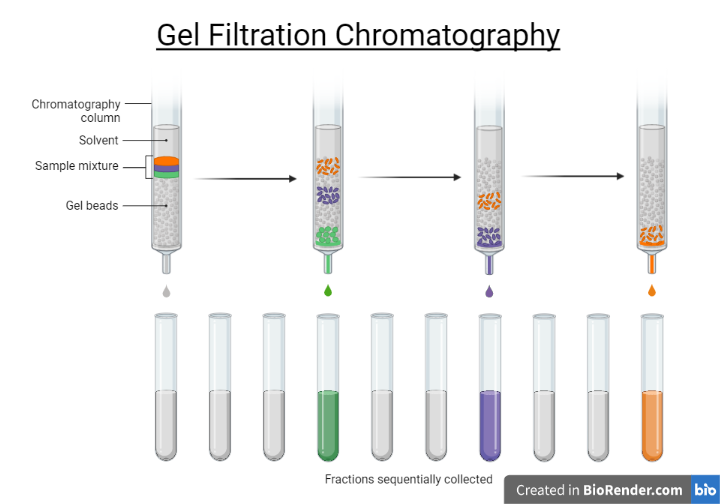
Fig: Size exclusion chromatography
Ion exchange chromatography
The attraction between solute ions and charged sites bound to the stationary phase is the basis of retention in ion-exchange chromatography. Ions with the same charge are not allowed to interact. Purification of water, ligand-exchange chromatography, protein ion-exchange chromatography, high-pH anion-exchange chromatography of carbohydrates and oligosaccharides, and other applications use this type of chromatography.
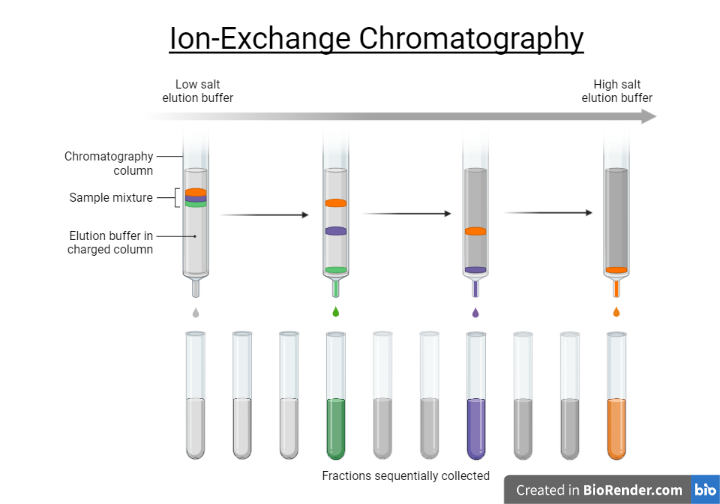
Fig: Ion-exchange chromatography
Affinity chromatography
Separation is based on reversible interactions between proteins and ligands. Ligands are covalently bonded to solid support on a bio-affinity matrix, which helps to keep proteins that interact with the column-bound ligands in place. There are two methods for eluting proteins coupled to a bio affinity column:
- Bio specific elution: Inclusion of a free ligand in the elution buffer that competes with the ligand attached to the column.
- A specific elution: The interaction protein with column-bound substrate is weakened by changes in pH, salt, and other factors.
Affinity chromatography can provide very high purity in a single stage due to the selectivity of the interaction.
Components:
- Mobile phase
- reservoir
- Pumps
- Injector
- Column
- Detector
- Data Acquisition & Control
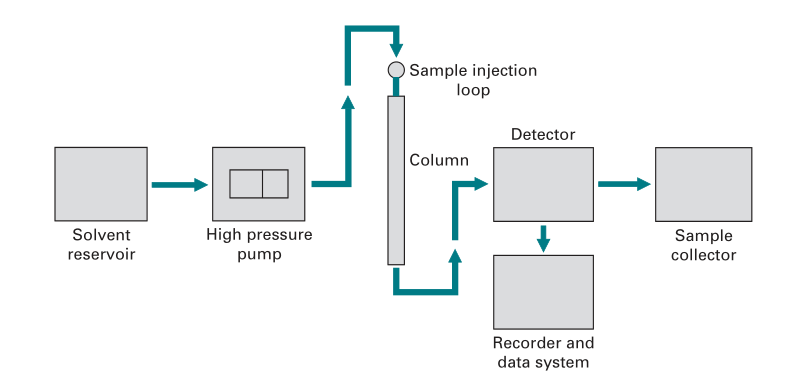
Fig: Components of a HPLC system
Columns
Conventional HPLC columns are typically constructed of stainless steel and designed to sustain pressures of up to 50 MPa.
Typical flow rates of 1-3 cm3 min–1 are achieved by using columns that are 3–25 cm long and have an internal diameter of about 4.6 mm.
Microbore or open tubular columns are 25-50 cm long and have an internal diameter of 1–2mm. They can maintain flow rates between 5 and 20 mm3 per minute.
Microbore columns have three important advantages over conventional columns:• Decreased eluent consumption as a result of slower flow rates
- Because of the lower flow rate, it’s perfect for using with a mass spectrometer.
- Improved sensitivity due to the ability to employ greater concentrations of analytes
Matrices and stationary phases
Based on a rigid solid structure, there are two basic types of matrix/stationary phase material. Both types use uniformly sized, roughly spherical particles to reduce the amount of area available for diffusion and thereby band widening. They’re generally made of styrene/divinylbenzene copolymers or chemically modified silica.
The two forms are:
- Microporous supports: Particles with a diameter of 5-10 mm have micropores
- Bonded phases: The stationary phase is chemically attached to an inert support, such as silica, in this type.
Mobile phases
The type of separation to be achieved determines the type of mobile phase to be employed in the separation.
- Isocratic elution can be performed by a single pump and a single eluent, or two or more eluents premixed in predetermined quantities.
- Separate pumps provide two eluents in quantities set by a gradient programmer in gradient elution.
Because traces of contaminants might influence the column and interfere with the detection system, all eluents for use in HPLC systems must be carefully purified.
Commercially available pure eluents for use in HPLC systems are available, however even with these, a 1-5 mm microfilter is usually used before the pump.
Gassing (the presence of air bubbles in the eluent) tends to occur in most pumps unless all eluents are degassed before use.
Gassing can affect column resolution and interfere with continuous eluate monitoring, which is seriously harmful for eluents including aqueous methanol and ethanol. Warming, magnetic stirring, vacuum, ultrasonication, and bubbling helium gas through the eluent reservoir are all strategies for degassing the eluent.
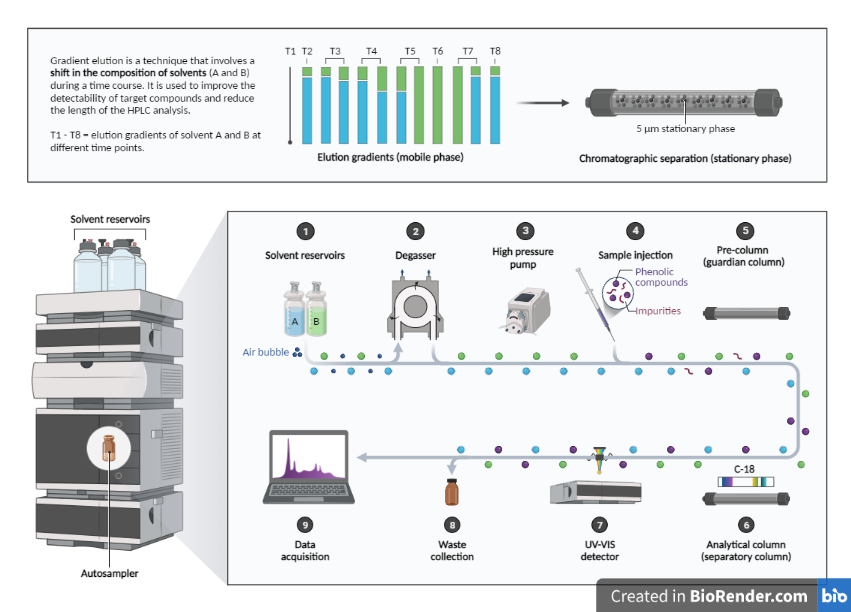
Fig: Components and Steps of High-performance Liquid Chromatography (HPLC) Analysis
Application of sample
The accurate application of the sample onto an HPLC column is a critical factor in achieving successful separations. A loop injector is the most prominent type of sample introduction.
This comprises of a metal loop that can be filled with the sample and has a fixed small volume. The eluent from the pump is then routed through the loop using a valve switching mechanism, and the sample is flushed onto the column via the loop outlet without disrupting the eluent flow to the column.
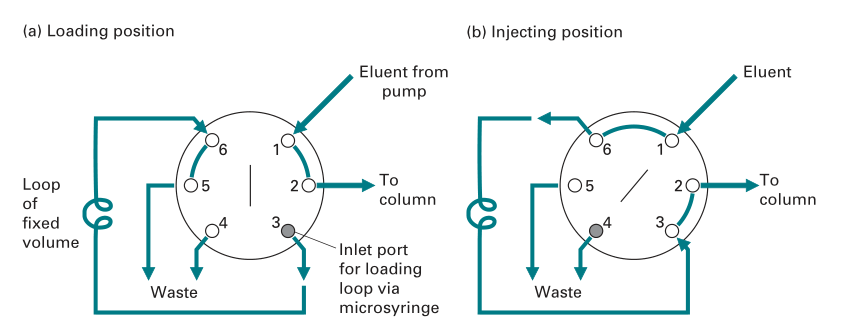
Fig: Loop injector used in HPLC
When very impure materials, such as sera, urine, plasma, or whole blood, are applied repeatedly, the column’s resolving power may be lost. In order to mitigate this limitation, a guard column is frequently built between the injector and the column. This guard column is a short (1-2 cm) column with the same internal diameter and packed with similar material as the analytical column. The packing in the guard column holds contaminated material preferentially and can be replaced at regular intervals.
Pumps
One of the most significant features of HPLC systems is the pumping system for supplying the eluent.
The primary characteristics of a suitable pumping system are that it can provide outputs of at least 50 MPa and that it should ideally have no pulses (i.e., cyclical pressure changes), as this can impair the detector response. For preparative separations, the flow capability must be at least 10 cm3 min-1 and up to 100 cm3 min–1.
Regardless of changing circumstances within the column, constant displacement pumps maintain a constant flow rate across the column. The most popular type of constant displacement pump is the reciprocating pump. Small pulses of flow are produced by such pumps, and pulse dampeners are frequently included in the system to reduce the pulsing impact.
All constant displacement pumps feature built-in safety cut-out systems that immediately deactivate the pump if the pressure within the column exceeds pre-determined limitations.
Detectors
Because the amount of material supplied to an HPLC column is usually very small, the detector system’s sensitivity must be high and reliable enough to respond to the low concentrations of each analyte in the eluate.
The most commonly used detectors are:
Variable wavelength detectors
These are based on spectrophotometry in the ultraviolet–visible range. These detectors can detect absorbances as low as 190 nm and provide full-scale deflection for as few as 0.001 absorbance units. They have a linear range of 105 and a detection sensitivity on the order of 5X 10–10 g cm–3. Continuous flow cells with a small internal capacity (usually 8 mm3) and an optical path length of 10 mm are used in all spectrophotometric detectors to allow continuous monitoring of the column eluate.
Scanning wavelength detectors
These have the capability of recording the entire absorption spectra of each analyte, which helps with identification. Such opportunities can be obtained by temporarily interrupting the eluent flow or by employing diode array techniques, which allow a scan of the entire spectrum of the eluate in less than 0.01 seconds and real-time display of the spectrum as a 3D graphic on a VDU screen.
Fluorescence detectors
Because of their higher sensitivity (10–12 g cm–3) than UV detectors, these are highly useful for HPLC. The approach, however, is hampered by the fact that only a few analytes glow. The technique’s uses can be expanded by pre-derivatizing the test sample.
Electrochemical detectors
These are electroactive analyte specific and potentially very sensitive. There are two types: amperometric and coulometric, both of which work on the same principles. Two electrodes, a steady counter electrode and a working electrode, are installed in a flow cell.
As analyte passes through the flow cell, a constant voltage is given to the working electrode, causing molecules of the analyte at the electrode surface to undergo either oxidation or reduction, resulting in current flow between the two electrodes.
The chromatogram is created by measuring the current’s size. The current observed causes a full-scale deflection on the recorder within the working analyte range, therefore the voltage given to the counter electrode is sufficient.
Applications:
HPLC has two main goals: preparative HPLC and analytical HPLC.
The process of isolating, purifying, identifying, quantifying, and resolving a compound is known as preparative HPLC. Analytical HPLC, on the other hand, is concerned with obtaining information about the sample compound.
Chemical Separations
Because various compounds migrate at varying rates depending on the column and mobile phase, the extent or degree of separation is largely governed by the stationary phase and mobile phase used.
Purification:
Purification is the separation or extraction of a target compound from a mixture of chemicals or contaminants. Under specific chromatographic conditions, each component showed a distinctive peak. The movement of the compounds and contaminants down the column must be sufficiently different so that only the pure target chemical may be collected or extracted without the presence of any unwanted compounds.
Identification
Compounds are typically assayed using HPLC. The assay parameters should be set so that the chromatograph shows a clear peak of the known sample. At the detection levels at which the assay will be run, the identifying peak should have a reasonable retention period and be well separated from unnecessary peaks.
Pharmaceutical applications
- A study of the dissolving of pharmaceutical dose forms in tablet form.
- Determining the shelf-life of pharmaceutical products
- Identifying the active components in dosage forms
- Assurance of pharmaceutical quality Applications in the environment
- Detection of phenolic chemicals in drinking water
- Diaphenhydramine identification in sedimented samples
- Pollutant bio-monitoring
Forensics
- Ensuring that soft drinks and drinking water are of good quality.
- Sugar content of fruit juices.
- Polycyclic compound analysis in vegetables.
- Military high explosives trace analysis in agricultural crops
Clinical
- HPLC offers a wide range of uses in science, including drug testing, pharmaceutical development, and nutrient analysis of blood serum in medicine.
- Through reliable quantitative determination of glycated hemoglobin, HPLC has proven to be a useful monitoring tool for glycemic control.
- On a commercial basis, evaluating medications
- It’s used to keep track of pollution levels in the environment.
- Target analytes for the HPLC Lab to detect and quantify are found in pesticides that protect crops from weeds and pests.
- It’s possible to count the quantity of contaminants in a drug sample down to the tenths of a percent.
- Genetic abnormalities are detected through biochemical tests.
Food and Flavor
- Ensuring the quality of soft drink and drinking water.
- Sugar analysis in fruit juices.
- Analysis of polycyclic compounds in vegetables.
- Trace analysis of military high explosives in agricultural crops.
References:
- Malviya, R., Bansal, V., Pal, O.P. and Sharma, P.K., 2010. High performance liquid chromatography: a short review. Journal of global pharma technology, 2(5), pp.22-26.
- Wilson, K., Hofmann, A., Walker, J.M. and Clokie, S. eds., 2018. Wilson and Walker’s principles and techniques of biochemistry and molecular biology. Cambridge University Press.
- Snyder, L.R., Kirkland, J.J. and Glajch, J.L., 2012. Practical HPLC method development. John Wiley & Sons.
- Ahuja, S. and Rasmussen, H. eds., 2011. HPLC method development for pharmaceuticals. Elsevier.
