Introduction
- Epigenetics is defined as heritable changes in gene expression that are, unlike mutations, not attributable to alterations in the sequence of DNA. The word “epigenetic” literally means “in addition to changes in genetic sequence.” The term has evolved to include any process that alters gene activity without changing the DNA sequence, and leads to modifications that can be transmitted to daughter cells (although experiments show that some epigenetic changes can be reversed).
- Despite not directly altering the DNA sequence, epigenetic mechanisms can regulate gene expression through chemical modifications of DNA bases and changes to the chromosomal superstructure in which DNA is packaged. It is the study of how cells control gene activity without changing the DNA sequence. “Epi-“means on or above in Greek, and “epigenetic” describes factors beyond the genetic code. Unlike genetic changes, epigenetic changes are reversible and do not change your DNA sequence, but they can change how your body reads a DNA sequence.
- Epigenetic changes are modifications to DNA that regulate whether genes are turned on or off. These modifications are attached to DNA and do not change the sequence of DNA building blocks. Because epigenetic changes help determine whether genes are turned on or off, they influence the production of proteins in cells.
- It consists of biochemical changes to the DNA or its associated proteins or RNA that does not change the DNA sequence itself but does impact the level of gene expression. This regulation helps ensure that each cell produces only proteins that are necessary for its function. For example, proteins that promote bone growth are not produced in muscle cells. Patterns of epigenetic modification vary among individuals, in different tissues within an individual, and even in different cells within a tissue.
- Epigenetic modifications can be maintained from cell to cell as cells divide and, in some cases, can be inherited through the generations.
Fig: Epigenetics
How Does Epigenetics Work?
Epigenetic changes affect gene expression in different ways. Types of epigenetic changes include:
DNA Methylation
DNA methylation works by adding a chemical group to DNA. Typically, this group is added to specific places on the DNA, where it blocks the proteins that attach to DNA to “read” the gene. This chemical group can be removed through a process called demethylation. Typically, methylation turns genes “off” and demethylation turns genes “on.” This is the addition or removal of a methyl group (CH3), predominantly where cytosine bases occur consecutively. DNA methylation was first confirmed to occur in human cancer in 1983, and has since been observed in many other illnesses and health conditions.
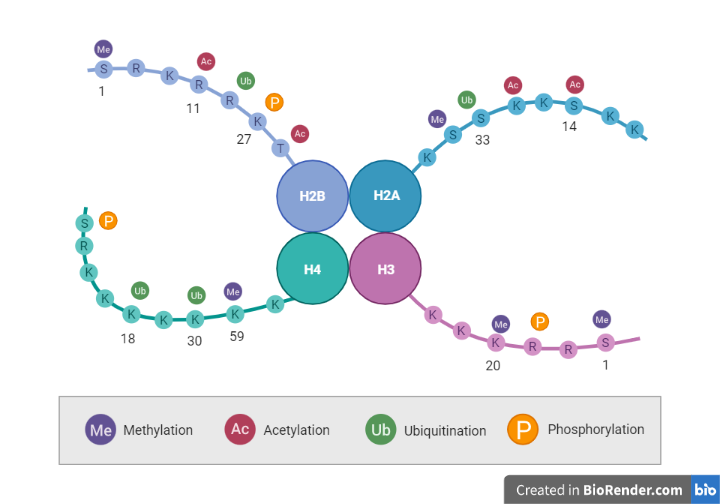
Fig: DNA Methylation
Histone modification
DNA wraps around proteins called histones. DNA wrapped tightly around histones cannot be accessed by proteins that “read” the gene. Some genes are wrapped around histones and are turned “off” while some genes are not wrapped around histones and are turned “on.” Chemical groups can be added or removed from histones and change whether a gene is unwrapped or wrapped (“on” or “off”).
Another significant epigenetic process is chromatin modification. Chromatin is the complex of proteins (histones) and DNA that is tightly bundled to fit into the nucleus. The complex can be modified by substances such as acetyl groups (the process called acetylation).
Briefly, negatively charged DNA is packaged around a positively charged histone protein octamer, which contains 2 copies of histone proteins H2A, H2B, H3, and H4. This nucleoprotein complex is a nucleosome, the basic unit of chromatin. The nucleosomes of a continuous DNA polymer are connected by linker DNA and the complex is stabilized by histone protein H1. The aggregation of chromatin results in the formation of a chromosome.
The chromatin of a chromosome exists as either loose, transcriptionally active euchromatin or dense, transcriptionally inactive heterochromatin. Chemical alterations to histone proteins can induce the formation of either the open euchromatin state, which facilitates gene expression by allowing transcription factors and enzymes to interact with the DNA, or the closed heterochromatin state, which suppresses gene expression by preventing initiation of transcription.
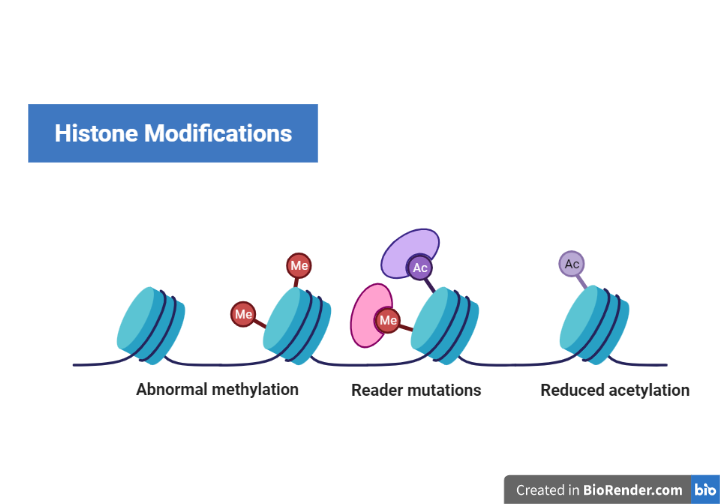
Fig: Histone Modifications
How Can our Epigenetics Change?
Your epigenetics change as you age, both as part of normal development and aging and in response to your behaviours and environment.
Epigenetics and Development
Epigenetic changes begin before you are born. All your cells have the same genes but look and act differently. As you grow and develop, epigenetics helps determine which function a cell will have, for example, whether it will become a heart cell, nerve cell, or skin cell.
Example: Nerve cell vs. Muscle cell
Our muscle cells and nerve cells have the same DNA but work differently. A nerve cell transports information to other cells in your body. A muscle cell has a structure that aids in your body’s ability to move. Epigenetics allows the muscle cell to turn “on” genes to make muscle proteins and turn “off” when not required.
Epigenetics and Age
Our epigenetics changes throughout your life. Our epigenetics at birth is not the same as your epigenetics during childhood or adulthood.
Example: Study of new-born vs. 26-year-old vs. 103-year-old
DNA methylation at millions of sites were measured in a new-born, 26-year-old, and 103-year-old.
The level of DNA methylation decreases with age.
A new-born had the highest DNA methylation, the 103-year-old had the lowest DNA methylation, and the 26-year-old had a DNA methylation level between the new-born and 103-year-old.
Epigenetics and Reversibility
Not all epigenetic changes are permanent. Some epigenetic changes can be added or removed in response to changes in behaviours or environment.
Example: Smokers vs. non-smokers vs. former smokers
Smoking can result in epigenetic changes. For example, at certain parts of the AHRR gene, smokers tend to have less DNA methylation than non-smokers. The difference is greater for heavy smokers and long-term smokers. After quitting smoking, former smokers can begin to have increased DNA methylation at this gene.
Eventually, they can reach levels similar to those of non-smokers. In some cases, this can happen in under a year, but the length of time depends on how long and how much someone smoked before quitting.
Key Words:
References:
- Paro, R., Grossniklaus, U., Santoro, R. and Wutz, A., 2021. Introduction to epigenetics (p. 215). Springer Nature.
- Hassler, M.R. and Egger, G., 2012. Epigenomics of cancer–emerging new concepts. Biochimie, 94(11), pp.2219-2230.
- Ballestar, E., 2011. An introduction to epigenetics. Epigenetic Contributions in Autoimmune Disease, pp.1-11.
- Van Soom, A., Peelman, L., Holt, W.V. and Fazeli, A., 2014. An introduction to epigenetics as the link between genotype and environment: a personal view. Reproduction in Domestic Animals, 49, pp.2-10.
- Dupont C, Armant DR, Brenner CA. Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med. 2009 Sep;27(5):351-7. doi: 10.1055/s-0029-1237423. Epub 2009 Aug 26. PMID: 19711245; PMCID: PMC2791696.
