Introduction:
- Enzyme inhibition is a process in which the activity of an enzyme is reduced or completely stopped by the presence of a molecule called an inhibitor.
- Enzyme inhibitors are molecules that interact with enzymes (temporary or permanent) in some way and reduce the rate of an enzyme-catalysed reaction or prevent enzymes to work in a normal manner.
- An enzyme inhibitor is a compound that affects the typical reaction pathway between an enzyme and a substrate.
- The binding of an inhibitor can stop a substrate from entering the enzyme’s active site and/or hinder the enzyme from catalysing its reaction
- All inhibitors may be combined in different groups in accordance with their chemical structure: ions of metals (Hg+, Fe2+, Cu+, Pb2+), organic compounds (e.g., N-ethylmaleimide, diisopropyl phosphofluoridate, oligomycin), and large bioorganic molecules, (peptides, proteins, etc).
- Many pharmacological drugs are enzyme inhibitors. The group of well-known pharmaceutical agents with name nonsteroidal anti-inflammatory drugs (NSAIDs) includes inhibitors of enzyme cyclooxygenase that catalyses a first step of synthesis of biologically active compounds prostaglandins that are responsible for the development of pain, inflammation, fever, contraction of smooth muscle, formation of blood clots, and others.
Types:
Inhibition binding is either reversible or irreversible.
Irreversible inhibition
It combines with the functional groups of the amino acids in the active site, irreversibly.
This type of inhibitors usually reacts with the enzyme and change it chemically (e.g., via covalent bond formation). Usually, these inhibitors modify the key amino acid residues needed for enzymatic activity
Examples:
Nerve gases and insecticides containing organophosphorus react with serine residues in the enzyme acetylcholine esterase.
Penicillin binds permanently to the active site of an enzyme termed DD-transpeptidase. The step in the formation of bacterial cell walls is performed by DD-transpeptidase. By blocking this enzyme, the bacteria are unable to form a cell wall and so cannot survive.
Irreversible inhibitors are covalently or noncovalently linked to the target enzyme and dissociate very slowly.
There are three types of irreversible inhibition:
- Group-specific reagents
- Reactive substrate analogs also known as affinity labels
- Suicide inhibition.
Group-specific reagents
Group-specific reagents, such as diisopropylphosphofluoridate (DIPF) and iodoacetamide, react with certain amino acid side chains.
DIPF, for example, modifies serine residues in chymotrypsin. This suggests that this particular residue is highly reactive; also, it is inferred that this particular residue is located in the active site of the enzyme chymotrypsin.
DIPF has also supplied data indicating the presence of a reactive serine residue within the active site of the enzyme Acetylcholinesterase via its interaction with active serine residues. DIPF and similarly structured molecules in acetylcholinesterase have inactivating functionality, which is indicative of a class of chemicals called as nerve agents.
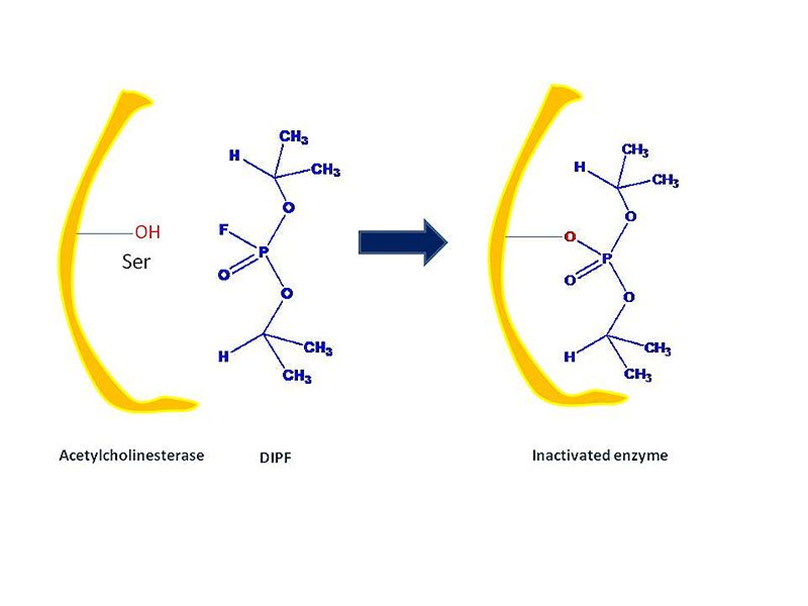
Fig: Example of Group-specific reagents types of irreversible inhibitor
Affinity labels (Reactive substrate analogs)
They are more particular than group specific reagents because they are structurally identical to the substrate that can covalently bind to the active site.
Tosyl-L-phenylalanine chloromethyl ketone (TPCK) is a chymotrypsin analog that binds to the active site and interacts irreversibly with the histidine residue to block the enzyme.
Another example is bromoacetol phosphate, which mimics dihydroxy acetone phosphate (DHAP) by binding covalently to the active site of Triose Phosphate Isomerase (TPI) and then permanently inhibiting the enzyme.
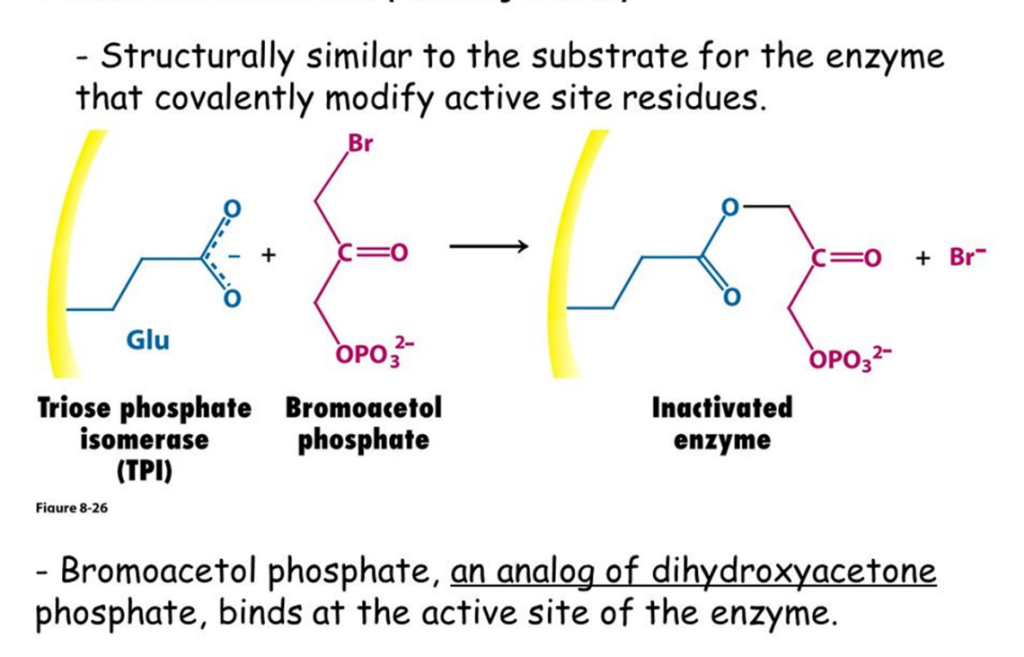
Fig: Example of affinity label types of irreversible inhibitor
Suicide inhibition
It is an irreversible form of enzyme inhibition that occurs when an enzyme binds a substrate analog and creates an irreversible complex with it via a covalent bond during the normal catalysis reaction. It is also known as suicide inactivation or mechanism-based inhibition.
The inhibitor binds to the active site, where it is changed by the enzyme to form a reactive group that irreversibly combines with the enzyme to form a stable inhibitor-enzyme complex.
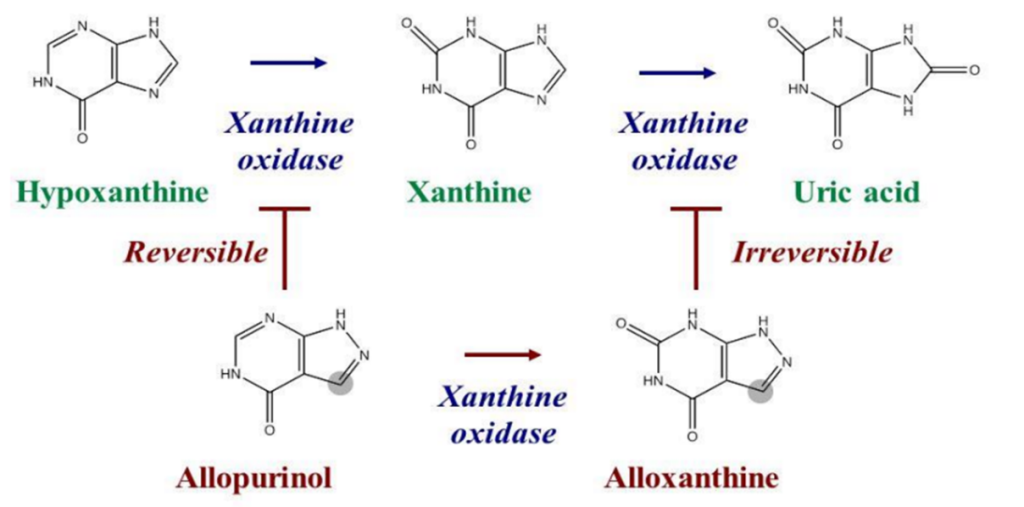
Fig: Example of suicide inhibition
Reversible Inhibition
Three kinds of reversible enzyme inhibitors which is classified according to the effect of varying the concentration of the enzyme’s substrate on the inhibitor.
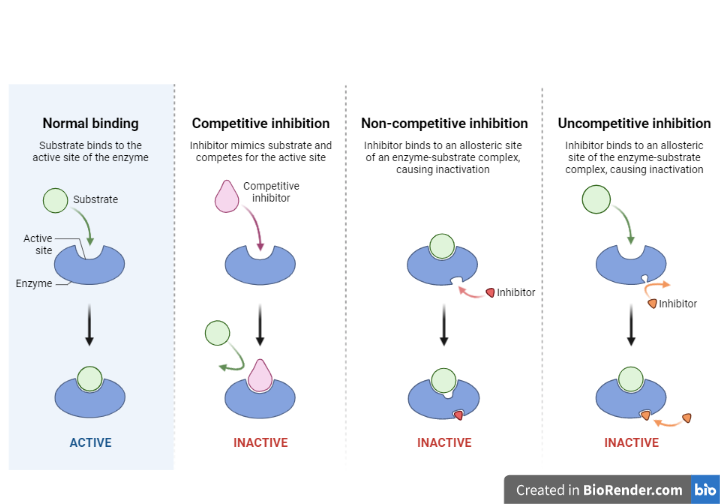
Fig: Different types of reversible inhibitors
Competitive inhibition
The inhibitor in competitive inhibition is a molecule that directly competes with a substrate for binding to the active site of the enzyme.
Competitive inhibitors are compounds that resemble substrates in structure and chemical characteristics and can attach to enzyme active sites.
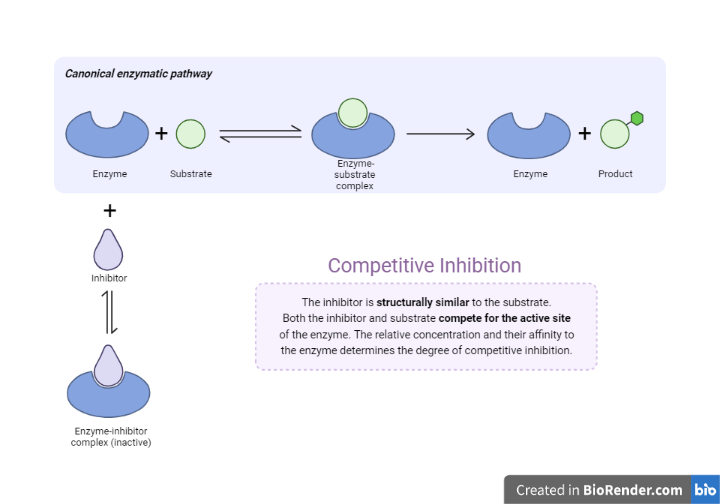
Fig: Competitive inhibition
Non-Competitive inhibition
Non-competitive inhibition is a type of enzyme inhibition in which the inhibitor inhibits enzyme activity while binding equally well to the enzyme whether or not it has already bound the substrate.
The inhibitor may bind to the enzyme whether or not the substrate has already been bound, but if it has a higher affinity for binding the enzyme in one state or the other, it is referred to as a mixed inhibitor.
In mixed inhibition, the inhibitor attaches to an enzyme location other than the active site and causes a conformational change.
The concentration of the substrate has no effect on the non-competitive.
Inhibits by binding irreversibly to the enzyme but not at the active site.
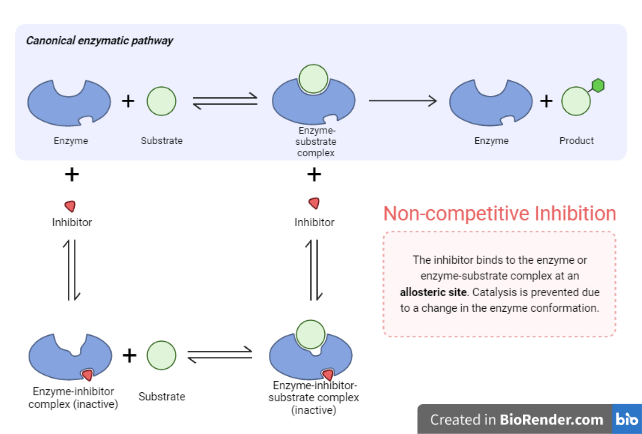
Fig: Non-Competitive inhibition
Examples
Cyanide combines with the iron in the enzyme’s cytochrome oxidase
Heavy metals, Ag or Hg, combine with –SH groups.
These can be removed by using a chelating agent such as EDTA.
Uncompetitive Inhibition
Binds at a different site than the active site on the substrate.
Unlike competing inhibitors, solely binds to the ES complex.
The inhibitor attaches to a place other than the substrate and is thus unaffected by the presence or absence of the substrate.
This action causes a conformational change in the protein, which disrupts a catalytic step and hence reduces or eliminates enzyme activity.
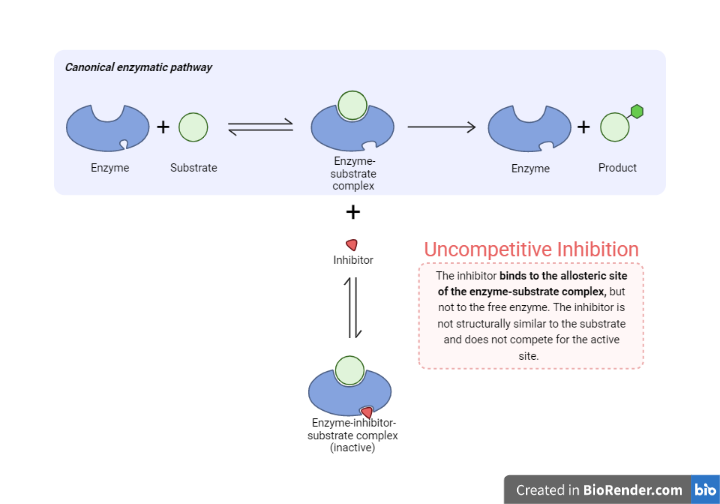
Fig: Uncompetitive inhibition
Applications:
- Understanding enzyme activity regulation in live cells
- Elaboration of cellular metabolic processes through intermediate accumulation
- Enables in the identification of catalytic or functional groups present at the active site of an enzyme.
- assists in providing information on the substrate specificity of enzymes.
- Supports in the investigation of catalytic activity mechanisms.
- Competitive or suicide inhibitors can also be used therapeutically.
References:
- Zollner, H., 1989. Handbook of enzyme inhibitors.
- Barman, T.E., 1969. Enzyme handbook (Vol. 1, p. 232). Berlin: Springer.
- Jencks, W.P., 1987. Catalysis in chemistry and enzymology. Courier Corporation.
- Satyanarayana, U., 2021. Biochemistry, 6e-E-book. Elsevier Health Sciences.
- Gerhart, J.C. and Pardee, A.B., 1962. The enzymology of control by feedback inhibition.
- David, L., Nelson, D.L., Cox, M.M., Stiedemann, L., McGlynn Jr, M.E. and Fay, M.R., 2000. Lehninger principles of biochemistry.
