Introduction:
It is a type of cancer immunotherapy treatment that controls the ability of T cells (an immune cells) to detect and target specific antigen-expressing cells, including cancer cells.
Moreover, this treatment involves genetic engineering of the T-cells of a patient to recognize and in order to attack cancer cells accordingly. In fact, the engineered T cells express a synthetic receptor which will binds a tumour antigen.
This therapy represents one of the major advancements in personalized cancer treatment.
Components:
It consists of
- A single-chain variable fragment (scFv), an extracellular ligand binding domain which is responsible for tightly binding to a tumour antigen
- A spacer
- A transmembrane region
- Intracellular costimulatory domain which is attached to an extracellular domain that transduces the responsible signal to trigger the signaling cascade pathway.
Mechanism:
T cells are a type of immune white blood cells which is known as lymphocytes that play a prominent role in adaptative immune response. It searches and fight illness, germs, and infection throughout the body.
A receptor which is presence in a T cell is used to recognises antigen and eventually destroy it after triggering and inducing several immunological responses.
However, the antigens that are present in the cancer cell are not detectable by the body cell as an abnormal. As a result, the cancer cells are skip from the immune system. In other words, T cells are not able to recognise and clear cancer cells.
In this scenario, CART-cells are genetically engineered cells that are designed in a laboratory in such a way that new receptor are induced into it so that they can easily bind to the cancer cell and can destroy them.
Different forms of cancer have distinct antigens. Each type of CAR T cell treatment is designed to target a specific type of cancer antigen. As a result, a CAR T cell therapy designed for one type of cancer will be ineffective against another.
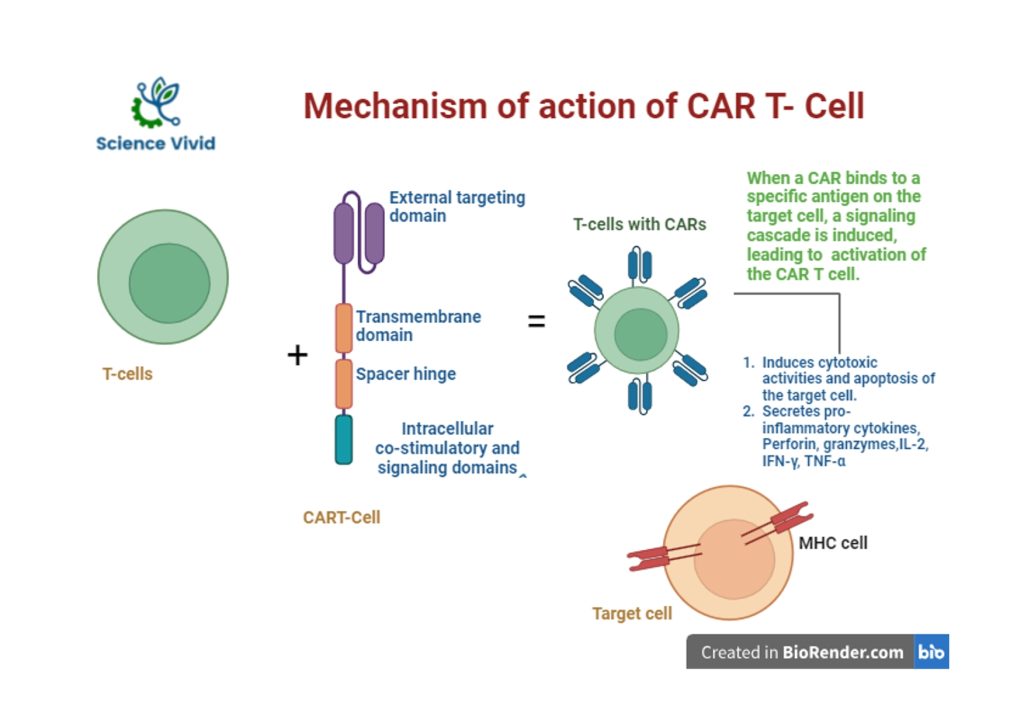
Fig: Mechanism action of Chimeric Antigen Receptor (CAR)-T cell therapy
Manufacturing of CAR T cell:
Collection of T- Cells
Manufacturing of CAR T cell therapy involves a series of procedure. Initially, a blood is drawn from the patient through an intravenous mode undergoing leukapheresis. White blood cells (including T cells) separated from the whole blood through a machine.
Engineering of the T- Cells
Furthermore, the separated T cells are activated and genetically modified using viral vectors to express chimeric antigen receptors (CARs) on their surface. The viral vectors, such as lentivirus or retrovirus introduced the gene for the CAR into the respective T cells and they are eventually cultured in a laboratory in order to increases their number and are cryopreserved (frozen) used.
Infusing the CAR T Cells
Furthermore, the prepared CART cells are processed and infused to the respective patient. Once infused, CAR T cells can recognize and destroy cancer cells that express the target antigen identified by the CAR.
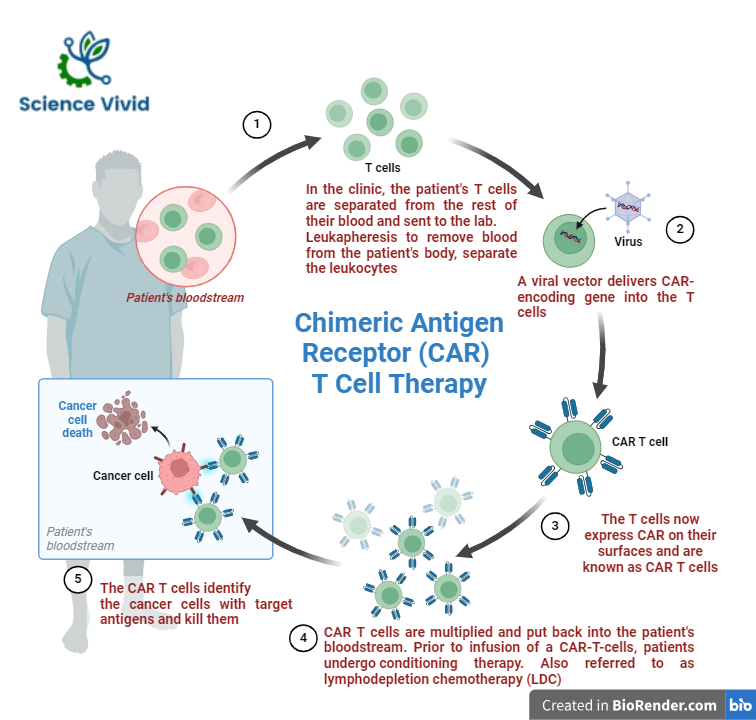
Fig: Manufacturing of Chimeric Antigen Receptor (CAR)-T cell therapy
FDA Approved CAR T-Cell Therapies:

Source: https://www.cancer.gov/
Potential side effects:
- High fever and chills
- Severe nausea, light-headedness or headaches
- Fatigue, tiredness
- Difficulty in speaking and understanding
- Pain in the muscles or joints
- Nausea, vomiting or diarrhoea
- Rapid heartbeat
- Low blood pressure
- Difficulty in breathing
- Muscle pain
- Confusion
- Tremors (twitching) or seizures
- Risk of infections, bruising or bleeding in blood
- Allergic reactions
