Basic parts:
A pH meter is a laboratory device that measures the acidity or alkalinity of a solution. pH is the unit of measure that the degree of acidity or alkalinity of the any given test solution that is measured on a scale of 0 to 14.
The PH meter consist of three basic parts:
- A glass bulb electrode, contain a solution of a certain fixed PH or hydrogen ion concentration
- A reference electrode, which is usually a calomel electrode
- A sensitive meter or measure device
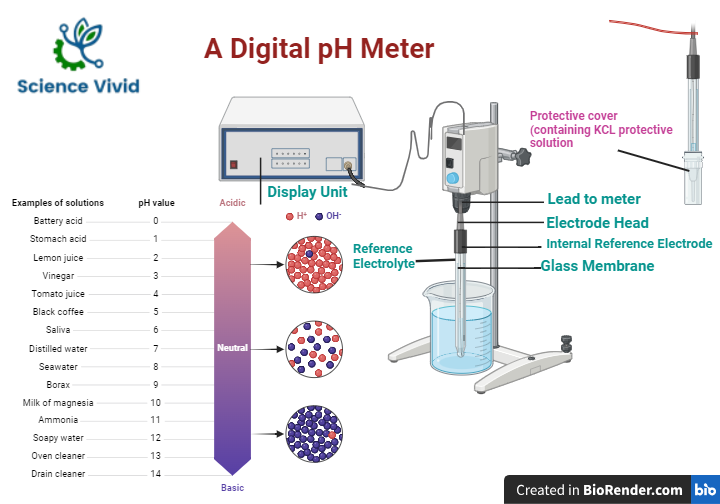
Fig: pH Meter Calibration
Measuring pH:
pH is defined as negative logarithm of H+ ion concentration. pH meter is an electric device and routinely used in laboratory that is used to measure concentration of hydrogen (H+) ions which reveal whether the given solution is acidic, alkaline or neutral in its nature on the basis of the pH value.
Principle:
pH meter designed in such a way that; it can measure the effective concentration of H+ ions in solution. A typical pH meter can measure the potential difference i.e., Electro Motive Force (EMF) which is developed between selective glass electrode and test solution containing H+
ions. The magnitude of this EMF varies with the varying temperature of the solution. The output potential is measured in millivolts [mv] and recorded glavanometrically or digitally on a pH-graduated scale.
V= E0+2.303RT/F=pH
Where, V= Voltage [observed EMF]
- E0= Potential of reference Electrode
- R= the gas constant [8.314 J/mole/oK]
- T= the absolute temperature in oK [25oC= 298 oK]
- F=the Faraday’s constant (964846 Coulombs/ equivalent weight or 9.64846 x 104 Coulombs mol-1)
Calibration of pH Meter:
Requirements
pH meter, Tissue paper, Magnetic stir plate and stir bar, Beakers, Commercial buffer capsules (pH 4.00, pH 7.00, pH 10.00 buffer), Distilled water, Deionized water
Preparation of calibration buffers
Prepare three different buffer solutions of pH 7.0, pH 4.0 and pH 9.0 by diluting the respective buffer capsules in a distilled water as instructed. Basically, dissolve the respective buffer capsule in an individual beaker containing 100 ml of distilled water using a glass rod to stir the buffer with moderate speed and uniform rate. Store it prior to calibration and allow all of the buffers to reach the same temperature because pH readings are temperature dependent. If the buffers are not at 25 °C, temperature compensation is highly recommended 7.0, pH 4.0 and pH 9.0 represent neutral, acidic and alkaline pH respectively.
pH meter standardization: (Electrode Preparation)
Prior to start calibration using the pH meter, wash the pH electrode gently with deionised water followed by wiping with tissue paper. Immerse the electrode in pH electrode storage solution, or 100 mL of pH 7 buffer with 0.5 g of potassium chloride (KCl).
Turn on the pH Meter
Switch on the pH meter and allow it to stabilize while conducting the calibration.
Calibration
- Firstly, immerse the electrode tip and junction into the first buffer solution (e.g., pH 7.00) and fully stir it at a moderate and uniform rate. Allow the pH reading to stabilize which will takes a few seconds to a minute.
- Wait for 1-2 minutes, if pH meter does not display the value of buffer automatically, gently set the value of buffer manually to pH 7.0. Then, mildly removed the electrode from pH 7 buffer solution and rinse the electrode with deionized water, later wipe/blot the electrode with tissue paper without touching the bottom membrane before moving to the next buffer solution.
Repeat for Additional Buffer Solutions
- Repeat the same steps of immersion, stabilization, adjustment, rinsing, and drying for each of the already prepared pH 4.0 followed by pH 9.0 buffer solutions.
- Now the pH meter has been standardized, and is ready for measuring the pH of given/test sample and do not alter any settings before measuring the unknown samples.
- Finally, after completion of the procedure, gently rinse and dry electrode and eventually return electrode to storage solution, turn off stir plate and clean up any mess.
Note
- The performance of pH calibration is always suggested prior to every use.
- It is highly recommended to extreme take care of the electrode because it is very fragile and expensive.
- Gently, rinse and dry electrode between each different solution immersion.
- The most common reason for errors in calibration errors because of the use of expired buffers. Make sure of its expiry and any contaminations
