Introduction to Type III Secretion System (T3SS):
The Type 3 Secretion System (T3SS), also known as injectisomes, is a complex structure made up of several subunits. It is present in a significant number of gram-negative bacteria and is composed of about 20 bacterial proteins with copy counts varying from 1 to more than 100. These proteins make up the T3SS machinery and are referred to as structural proteins. Other proteins known as “translocators” have the job of moving a different group of proteins into the cytoplasm of the host cell. We refer to the translocated proteins as “effectors or effector molecules.” One of the most intricate membrane-localized molecular machinery known is the virulence-associated T3SS, which facilitates the injection of bacterial effector proteins into eukaryotic host cells.
Mechanism of Protein Secretion:
Despite their structural diversity and wide range of functions, secreted effectors are all released in an unfolded conformation by a single, ATP- and proton-motive force (PMF)-driven mechanism that involves an N-terminal secretion signal and frequently also cognate targeting chaperones. T3SS substrate secretion is typically believed to be a one-step process, but Yersinia has lately called into question this idea. T3SSs have ten to fifteen more proteins in addition to the nine core proteins that are either necessary or significant to their function.
Typically, a limited number of operons found on plasmids or in pathogenicity islands within the bacterial chromosome encode the structural components of T3SSs. Bacteria that are evolutionarily dissimilar may have closely related systems since T3SSs are usually horizontally acquired, and vice versa. Shigella and E. coli, for instance, have very similar genomes; nevertheless, Shigella T3SS is more similar to Salmonella T3SS than it is to the Enteropathogenic E. coli (EPEC) and Enterohemorrhagic E. Coli (EHEC) disease systems. Seven families of T3SSs have been proposed, primarily based on the similarities between their extracellularly enlarged needles, tips, and translocons.
The entire system can be roughly divided into: Cytoplasmic components that make up the basal body, which spans the inner and outer membranes to form a structure like a socket with a central rod and several rings. There are at least fifteen proteins in it. ATPase activity and substrate selectivity are facilitated by cytoplasmic components. When ATP is triggered, the export apparatus forces protein across the whole channel while the memory components hold the inner rod in place. The needle is a filament that emerges from the basal body and is encased in it. It penetrates the extracellular space and the secretin. Its inner hollow core is sufficiently wide to allow for the passage of an unfolded effector.
Translocon and Host Cell Interaction:
The translocon, a T3SS tip complex found on the needle’s outer end, is essential for detecting host cell contact and controlling effector secretion. Additionally, the translocon must integrate into the membranes of the host cells. While effector secretion outside of the bacterium does not require the T3SS translocon, effector transit across host cell membranes must. When translocons come into contact with host cells, they come together to form a pore that is necessary for the transport of effectors.
However, a different two-step model of Type 3 effector translocation has recently been put forth, in which effectors and translocon components are produced before host cell contact and stay attached to the bacteria, possibly in lipid vesicles. The translocon and tip proteins create a channel through which the effectors transit following contact with host cells, possibly detected by the needle.
Instances related to significance:
- By invading nonphagocytic cells and killing macrophages, Salmonella enterica serovars cause intestinal inflammation and bacteremia in cows (serovar Dublin), septicemia in pigs (serovar Choleraesuis), enteric fever in humans (serovar Typhi, Paratyphi, and Sendai), and typhlitis and typhoid-like disease in mice (serovar Typhimurium).
- Shigella species (S. dysenteriae, S. flexneri, S. boydii, and S. sonnei) produce human dysentery pandemics and Bacillary dysentery (shigellosis).
- Phagocytosis is inhibited by Yersinia species (Yersinia pestis, Yersinia enterocolitica, and Yersinia pseudotuberculosis), which cause mesenteric lymphadenitis and enterocolitis, and plague (bubonic, pneumonic, and septicemic) (Yersinia pestis).
- In tight adhesion mediation (EPEC)
Structural Components of Type III Secretion System (T3SS):
An overview of the T3SS model using Yersinia species as an illustration.
It is made up of the following
- Basal body and Cytoplasmic components
- YscQ family members’ predicted C ring, YscV family members’ cytoplasmic domain (which serves as a docking site for T3SS substrates), and YscN family members’ hexameric ATPase are examples of cytoplasmic components. ATP is not cleaved if bacteria are unable to identify the substrate. The YscU family’s cytoplasmic domain is involved in substrate selection.
- For the membrane-spanning component, there are two types of rings.
The outer membrane of the Ysc family (secretin) holds in place the inner membrane ring of the YscC and YscD families, which also contain two proteins linked to the inner rod.
Export-related equipment: The five hydrophobic proteins that make up the T3SS export apparatus are highly conserved throughout injectisomes (YscU, V, R, S, and T families). - Needle in animal pathogens (40-80nm long), pilus in plant pathogens (upto 2 µm long for spanning cell wall)
- Pore complex: YopB and YopD families’ heterooligomeric translocon (6–8 subunits) and the LcrV family’s pentameric tip complex. The creation of a translocon complex allows for penetration of the host cell membrane.
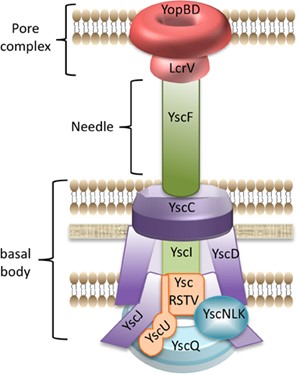
Fig: Type III secretion system in Yersinia
References:
- Green, E. R., & Mecsas, J. (2016). Bacterial Secretion Systems: An Overview. Microbiology spectrum, 4(1), 10.1128/microbiolspec.VMBF-0012-2015. https://doi.org/10.1128/microbiolspec.VMBF-0012-2015
- Wagner, S., Grin, I., Malmsheimer, S., Singh, N., Torres-Vargas, C. E., & Westerhausen, S. (2018). Bacterial type III secretion systems: a complex device for the delivery of bacterial effector proteins into eukaryotic host cells. FEMS microbiology letters, 365(19), fny201. https://doi.org/10.1093/femsle/fny201
