Introduction:
The Philadelphia chromosome (Ph chromosome) is a specific genetic abnormality found in certain types of cancer, most notably chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL).
The Ph chromosome is a reciprocal translocation between chromosomes 9 and 22, in which a small piece of chromosome 22 breaks off and attaches to chromosome 9.
This translocation leads to the formation of a new gene, BCR-ABL1, which encodes a tyrosine kinase enzyme that promotes the uncontrolled growth of cancer cells. The BCR-ABL1 gene is present in more than 95% of CML cases and a smaller percentage of ALL cases.
In 1960, Nowell and Hungerford identified the Philadelphia chromosome as the first tumour-specific chromosomal alteration. It was given the name of the town where it was found.

Fig: The Philadelphia Chromosome
Pathogenesis:
The Philadelphia chromosome was the first recurrent genetic change linked to a specific human malignancy, chronic myeloid leukemia (CML). It was discovered as an unusually tiny chromosome in CML cells. Later, a chromosomal rearrangement involving the ABL gene on chromosome 9, a tyrosine kinase, and the BCR gene on chromosome 22 was discovered. It is suspected that the BCR-ABL fusion gene causes uncontrolled cell growth. There are numerous methods for detecting the Philadelphia chromosome, which can be used to diagnose CML and track a patient’s clinical progress.
CML and Philadelphia chromosome-positive ALL are leukemias caused by a mutation known as the Philadelphia chromosome. The mutation is a translocation designated as t (9;22). (q34; q11). This aberrant chromosome has a fusion gene composed of the ABL and BCR genes, which results in the BCR-ABL oncogene. This oncogene expresses an enzyme with abnormal tyrosine kinase activity.
There are numerous pathways associated with the pathogenesis of BCR-ABL1 gene, including:
The Ras-Raf-MEK-ERK pathway: This pathway is activated by the BCR-ABL1 tyrosine kinase, which leads to the phosphorylation and activation of the proteins in this pathway. This leads to increased cell proliferation, survival and resistance to apoptosis.
The PI3K-Akt pathway: Activation of this pathway by BCR-ABL1 leads to increased cell proliferation, survival, and resistance to apoptosis.
The JAK-STAT pathway: Activation of this pathway by BCR-ABL1 leads to increased cell proliferation and survival, and also plays a role in the differentiation of leukemic cells.
The c-Myc pathway: Activation of this pathway by BCR-ABL1 leads to increased cell proliferation and survival and also plays a role in the formation of cancer stem cells.
The p53 pathway: BCR-ABL1 is a negative regulator of the p53 tumor suppressor protein, which leads to decreased cell cycle control and DNA repair, and increased risk of cancer.
The angiogenesis pathway: BCR-ABL1 promotes the formation of new blood vessels, which is important for the growth and spread of cancer cells.
The inflammasome pathway: BCR-ABL1 leads to the activation of inflammasome, which is a complex of proteins that plays a role in the inflammatory response and can contribute to the development of cancer.
The senescence pathway: BCR-ABL1 leads to the activation of senescence, which is a process of cell aging that can contribute to the development of cancer.
Leukemia:
Leukemia is a blood cancer that affects the bone marrow. There are different types of leukemia, classified based on the type of cell affected and the rate at which the disease progresses. Two main types of leukemia are acute and chronic. The Philadelphia Chromosome is a key genetic alteration in the development of Leukemia, specifically Chronic Myeloid Leukemia (CML) and Acute Lymphoblastic Leukemia (ALL) and it is identified by the presence of BCR-ABL1 gene which results in overproduction of a protein that promotes the growth of cancer cells.
Chronic myeloid leukemia (CML)
It is also known as chronic myelogenous leukemia, is a type of chronic leukemia that affects the cells in the bone marrow that eventually develop into white blood cells. CML is caused by the presence of the Philadelphia chromosome, which is a specific genetic alteration in which a piece of chromosome 9 breaks off and attaches to chromosome 22, forming a new, abnormal chromosome 22. This translocation results in the formation of a new gene, BCR-ABL1, which produces an abnormal protein called tyrosine kinase that promotes the growth of cancer cells.
Acute lymphoblastic leukemia (ALL)
This is a type of acute leukemia that affects the cells in the bone marrow that eventually develop into white blood cells. It is more frequent in youngsters, but it can occur in adults as well. It is characterized by the overproduction and accumulation of immature white blood cells in the bone marrow and blood. The presence of the Philadelphia chromosome and the BCR-ABL1 gene is found in about 30-35% of people with ALL.
Molecular Biology:
The Philadelphia chromosome is a result of a translocation between chromosomes 9 and 22, specifically a reciprocal translocation between the long arms of these chromosomes, t (9;22) (q34; q11). This translocation results in the formation of a new gene, BCR-ABL1, which is located on chromosome 22.
The BCR gene located on chromosome 22 codes for a protein called breakpoint cluster region protein, and ABL1 gene located on chromosome 9 code for a tyrosine kinase protein. The fusion of these two genes results in the formation of a new protein, BCR-ABL1, which is a constitutively active tyrosine kinase.
This protein disrupts normal cell signalling pathways by activating downstream signalling molecules, leading to uncontrolled cell growth and division, which is a hallmark of cancer.
Mechanism:
The BCR/ABL1 fusion protein is the result of a genetic translocation between chromosomes 9 and 22, which leads to the formation of the Philadelphia chromosome (Ph). The BCR/ABL1 gene encodes for a tyrosine kinase enzyme that is responsible for the development and progression of leukemia. The mechanism of BCR/ABL1 is as follows:
- The BCR/ABL1 fusion protein is constitutively active, meaning it is always turned on. This is due to the presence of a unique juxtamembrane domain in the BCR part of the fusion protein.
- The tyrosine kinase activity of the BCR/ABL1 protein leads to the phosphorylation of downstream target proteins, which in turn leads to the activation of signalling pathways that promote cell growth and survival.
- The BCR/ABL1 protein also induces resistance to apoptosis, which is the process of programmed cell death. This further contributes to the uncontrolled cell growth and division that is characteristic of cancer.
- BCR/ABL1 also modulates the expression of genes that are involved in cell cycle regulation, angiogenesis and inflammation.
- BCR/ABL1 is a negative regulator of the tumour suppressor protein p53, which is a gatekeeper of the cell cycle and plays a role in DNA repair.
- BCR/ABL1 tyrosine kinase also activates several signalling pathways, including the Ras-Raf-MEK-ERK, PI3K-Akt, and JAK-STAT pathways, which are all involved in cell proliferation and survival.
- Activation of these pathways can lead to the activation of transcription factors such as c-Myc, which can promote cell growth and division.
- BCR/ABL1 also plays a role in the formation of cancer stem cells, which are a subpopulation of cancer cells that are resistant to chemotherapy and radiation therapy and are thought to be responsible for cancer recurrence.
Clinical Significance:
The discovery of the Philadelphia chromosome, a genetic alteration that occurs in certain types of leukemia, has had a significant impact on the understanding and treatment of cancer and continues to shape the field of cancer research and treatment today.
The legacy of the Philadelphia chromosome includes the following:
Advancement in cancer treatment: The discovery of the BCR-ABL tyrosine kinase as the oncogenic driver of the Philadelphia chromosome-positive leukemias led to the development of BCR-ABL tyrosine kinase inhibitors (TKIs) such as Imatinib, Dasatinib, Nilotinib and Bosutinib, which have dramatically improved the treatment of chronic myeloid leukemia (CML).
Understanding the molecular basis of cancer: The Philadelphia chromosome provided the first clear demonstration that cancer can be caused by a specific genetic alteration and that targeting this alteration with a specific treatment can be effective.
Advancement in personalized medicine: The discovery of the Philadelphia chromosome and the development of TKIs has led to the emergence of personalized medicine in cancer treatment, where treatments are tailored to specific genetic alterations in the cancer cells.
Advancement in cancer research: The discovery of the Philadelphia chromosome has led to an increased understanding of the molecular mechanisms of cancer and has provided a foundation for the development of new cancer therapies.
Impact on the field of Genetics: The discovery of the Philadelphia chromosome has greatly increased the understanding of genetic changes in cancer and how they contribute to the development of the disease, which is essential to the field of genetics.
Advancement in the understanding of progression of cancer: The discovery of the Philadelphia chromosome and the development of TKIs has led to the understanding of how cancer progresses and how to target it at different stages of the disease.
Testing:
There are several types of tests available and it is important to note that the specific type of test used will depend on the individual case and the stage of the disease.
Polymerase chain reaction (PCR) test: This test is used to detect the presence of the BCR-ABL1 gene in a patient’s blood or bone marrow. It is considered the most sensitive test for detecting the mutation.
Fluorescence in situ hybridization (FISH) test: This test uses fluorescent dyes to detect the presence of the BCR-ABL1 gene in a patient’s blood or bone marrow. It is less sensitive than PCR but can be used to confirm the results of a PCR test.
Chromosome analysis: This test uses a microscope to look at the patient’s chromosomes and detect any abnormalities that may indicate the presence of the BCR-ABL1 gene.
Immunophenotyping:
This is also known as Qualitative BCR-ABL1 testing. This test uses antibodies to detect the presence of specific proteins that are associated with the BCR-ABL1 gene. The qualitative BCR-ABL1 test can determine the specific type (isoform) of the Philadelphia chromosome present, which is critical for accurate diagnosis and treatment. The qualitative BCR-ABL1 test reveals the existence of the p190, p210, and p230 isoforms; however, the qualitative test does not assess transcript levels.
Next-generation sequencing (NGS) test: This test is a high-throughput DNA sequencing method that can detect BCR-ABL1 gene mutation in the blood or bone marrow, this method is considered to be more sensitive and specific than the PCR test.
Specimens:
The specimens used for BCR-ABL1 testing typically include blood or bone marrow samples. It’s important to note that the specific type of specimen used will depend on the individual case, the stage of the disease, and the type of test being performed.
Blood sample: A small amount of blood is drawn from the patient and sent to the lab for testing. The blood is usually collected in a tube containing an anticoagulant to prevent it from clotting. The test can detect BCR-ABL1 mutation in the peripheral blood, it can be used as a monitoring tool to follow the response to treatment.
Bone marrow sample: A bone marrow sample is obtained by a procedure called a bone marrow aspiration and biopsy. During this procedure, a small amount of bone marrow is removed from the patient’s hip bone using a needle and sent to the lab for testing. Bone marrow samples are considered the gold standard for BCR-ABL1 testing, as they are more likely to contain cancerous cells than blood samples.
In addition, liquid biopsy test is also available, a liquid biopsy is a type of test that uses a sample of blood to detect cancer cells or genetic changes associated with cancer. This test can detect BCR-ABL1 mutation in the cell-free DNA (cfDNA) present in the blood.
The specimens really depend upon the test required:
BCR-ABL1 cytogenetic testing: 1-3 ml bone marrow OR peripheral blood in green (Na Hep) tube.
Qualitative BCR-ABL1 testing: 3 ml peripheral blood OR bone marrow in purple (EDTA) tube (Please note that samples must be received within 48 hours after collection).
Quantitative BCR-ABL1 testing: 5-10 ml peripheral blood or 3-5 ml bone marrow in purple (EDTA) tube (please note that samples must be received within 24 hours after collection).
Diagnostic Algorithm:
It’s important to note that this is a general diagnostic algorithm and may vary depending on the specific case, the stage of the disease, and the patient’s overall health.
Initial evaluation: This typically includes a medical history, physical examination, and laboratory tests such as a complete blood count (CBC) and peripheral blood smear.
Confirmation of leukemia: If initial evaluation suggests the presence of leukemia, a bone marrow aspiration and biopsy will be performed to confirm the diagnosis and to determine the type of leukemia.
BCR-ABL1 testing: Once the diagnosis of leukemia is confirmed, a BCR-ABL1 test will be performed on the bone marrow or blood sample to detect the presence of the BCR-ABL1 mutation.
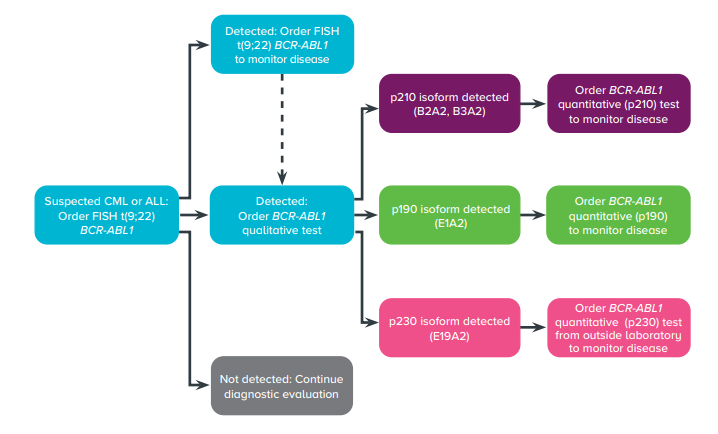
Fig: Diagnostic Algorithm
Molecular testing: In case of a positive BCR-ABL1 test, a molecular test, such as quantitative PCR, will be performed to measure the level of BCR-ABL1 transcript in the blood or bone marrow.
Additional testing: Additional tests may be performed to further determine the subtype of leukemia and to evaluate the patient’s overall health. These may include cytogenetic testing, fluorescence in situ hybridization (FISH) and next-generation sequencing (NGS).
Treatment: If the BCR-ABL1 mutation is detected, treatment will be initiated according to the type and stage of the disease, and patient’s overall health. Treatment options may include tyrosine kinase inhibitors (TKIs) and stem cell transplantation.
References:
- Maloy, S. and Hughes, K. eds., 2013. Brenner’s encyclopedia of genetics. Academic Press.
- Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009; 27:6041-6051.
- Baccarani, M., et al. (2013). European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood, 122(6), 872–884.
- Soverini, S., Bassan, R. & Lion, T. Treatment and monitoring of Philadelphia chromosome-positive leukemia patients: recent advances and remaining challenges. J Hematol Oncol 12, 39 (2019)
- American Society of Clinical Oncology. Leukemia – Chronic Myeloid – CML: Treatment Options. 11/2016. Accessed at www.cancer.net/cancer-types/leukemia-chronic-myeloid-cml/introduction on May 14, 2018.
- National Cancer Institute. Chronic Myelogenous Leukemia Treatment (PDQ)-Patient Version. March 30, 2018. Accessed at www.cancer.gov/types/leukemia/patient/cml-treatment-pdq on May 14, 2018.
- Laboratory, G. and G. D. Molecular Genetic Testing For BCR-ABL Fusion Gene. (2019).
