Introduction:
The DNS (3,5-dinitrosalicylic acid) method is commonly used for the quantitative estimation of reducing sugars, including glucose. This method is based on the reaction of reducing sugars with DNS reagent, which results in the formation of a coloured compound that can be measured spectrophotometrically.
Principle:
The principle behind the DNS (3,5-dinitrosalicylic acid) method for the quantitative estimation of glucose is based on the reaction between reducing sugars, such as glucose, and DNS reagent. In the presence of reducing sugars, DNS reagent undergoes a chemical reaction, resulting in the formation of a coloured compound.
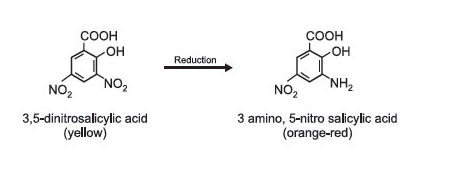
The DNS reagent contains 3,5-dinitrosalicylic acid, which is yellow in its oxidized form. When reducing sugars, including glucose, are present in the reaction mixture, they reduce the DNS reagent to 3-amino-5-nitrosalicylic acid, which is a red-coloured compound. The intensity of the red colour is directly proportional to the concentration of the reducing sugars, specifically glucose, in the sample.
By measuring the absorbance of the coloured compound at a specific wavelength using a spectrophotometer, the concentration of glucose in the sample can be determined. This is typically done by preparing a series of glucose standard solutions with known concentrations and constructing a calibration curve relating the absorbance values to the corresponding glucose concentrations. The absorbance of the sample is then compared to the calibration curve to determine its glucose concentration.
In summary, the DNS method exploits the reducing property of glucose to generate a coloured compound, and the intensity of the colour is used as a measure of glucose concentration.
Materials:
- Distilled water
- Test tubes or cuvettes
- Spectrophotometer
- Pipettes
- Bunsen burner or hot plate
Reagents Required:
- Sodium potassium tartrate (Rochelle salt solution): Dissolve 45 gms of sodium potassium tartrate in 75 mL of H2O.
- 3,5-DNS Reagent: Dissolve 1.5 gm of DNS reagent in 30 mL of 2 M/liter NaOH.
- Standard glucose solution (1mg/ml)
Procedure:
- Take several clean, dry and sterile test tubes.
- Pipette out standard sugar solution in the range of 0 to 3 mL in different test tubes and make up the volume of all test tubes to 3 ml with distilled water.
- .Add 1 ml DNS reagent to all the test tubes and mix plug the test tube with cotton or marble and keep the test tube in a boiling water bath for 15 minutes.
- .After cooling to room temperature in a cold-water bath, add 1 ml of a 40% potassium sodium tartrate (Rochelle salt) solution to stabilize the colour.
- Finally, record the absorbance with a spectrophotometer at 540nm.
- Please note that all the tubes must be cooled to room temperature before reading, since the absorbance is sensitive to temperature.
- Prepare standard curves of the sugars provided and use them to estimate the concentration of the unknowns provided.
| S. No | Volume (ml) of Standard glucose | Concentration of glucose | Volume (ml) of distilled water | Volume of DNS reagent ( ml) | 40% Rochelle salt solution | Absorbance (540nm) O.D. | |
| 1 | Blank | – | 3 | 1 | keep the test tube in a boiling water bath for 5-15 minutes | 1 ml | – |
| 2 | 0.3 | 100 ug/ml | 2.7 | 1 | keep the test tube in a boiling water bath for 5-15 minutes | 1 ml | – |
| 3 | 0.6 | 200 ug/ml | 2.4 | 1 | keep the test tube in a boiling water bath for 5-15 minutes | 1 ml | – |
| 4 | 0.9 | 300 ug/ml | 2.1 | 1 | keep the test tube in a boiling water bath for 5-15 minutes | 1 ml | – |
| 5 | 1.2 | 400 ug/ml | 1.8 | 1 | keep the test tube in a boiling water bath for 5-15 minutes | 1 ml | – |
| 6 | 1.5 | 500 ug/ml | 1.5 | 1 | keep the test tube in a boiling water bath for 5-15 minutes | 1 ml | – |
| Test solution A | Test solution (3ml) | – | – | 1 | keep the test tube in a boiling water bath for 5-15 minutes | 1 ml | – |
| Test solution B | Test solution (3ml) | – | – | 1 | keep the test tube in a boiling water bath for 5-15 minutes | 1 ml | – |
Results and Interpretation:
- Prepare a standard curve using known concentrations of glucose.
- Plot the absorbance values against the concentrations to create a linear standard curve.
- Use the standard curve to determine the concentration of glucose in your sample based on its absorbance.
- If you performed dilutions during the sample preparation, ensure to account for these dilutions in your final concentration calculation.
Note: Ensure that proper safety precautions are followed while working with chemicals and heat sources.
