Introduction:
A qualitative test for amino acids is a laboratory test that is used to identify the presence of a specific amino acid or a group of amino acids in a sample. The test typically involves the use of a reagent that reacts with the amino acid(s) of interest, resulting in a color change or precipitation.
General Tests:
Solubility test
A solubility test for amino acids is a laboratory test that is used to determine the solubility of an amino acid in different solvents. The solubility of an amino acid can be an indicator of its chemical properties and can be useful in the identification of unknown compounds.
To perform a solubility test, a small amount of the amino acid sample is added to a test tube containing a known volume of the solvent of interest. The test tube is then shaken or stirred to ensure that the amino acid is in contact with the solvent. The solubility of the amino acid can then be determined by observing whether the amino acid dissolves or remains in a solid form.
It is important to note that some amino acids have different solubility properties depending on the pH of the solution. Additionally, some amino acids are insoluble in most solvents, so more advanced techniques such as chromatography may be required for their detection.
Ninhydrin test
The Ninhydrin Test is a qualitative test used to detect the presence of amino acids, specifically the alpha-amino group. The test is based on the reactivity of the alpha-amino group with Ninhydrin (2,2-dihydroxyindane-1,3-dione), a compound that forms a complex with the amino acid and results in a colour change.
Reagents:
The Ninhydrin reagent is composed of Ninhydrin (2,2-dihydroxyindane-1,3-dione) dissolved in a suitable solvent, typically ethanol or methanol. The concentration of Ninhydrin in the reagent can vary, but it is typically in the range of 0.1-1%.
The reagent also contains a small amount of an acid, such as acetic acid or hydrochloric acid, to adjust the pH of the solution and to promote the formation of the coloured complex between Ninhydrin and the amino acid. It can also contain other auxiliary reagents such as mercuric acetate, to improve the sensitivity of the test.
Procedure
- Take a small volume of test sample and place it in a test tube.
- Add a few drops of the Ninhydrin reagent to the test tube containing the amino acid sample.
- Heat the mixture in a water bath at a temperature of around 100-120°C for 5-10 minutes.
- Observe the colour change of the solution, a positive result will show a purple color indicating the presence of an alpha-amino group in the sample.
- Compare the results with a known positive control sample.
Biuret test
Principle
This test is used to determine the presence of peptide bonds in a protein sample.
The test is performed by adding a small amount of protein sample to a solution of copper sulfate, which is usually blue in colour. If the sample contains peptide bonds, the complex that forms will absorb the blue light, causing a shift in the colour of the solution from blue to green or blue-green. The intensity of the colour change is directly proportional to the amount of protein present in the sample.
Reagents
The Biuret reagent is typically composed of copper (II) sulfate pentahydrate (CuSO4·5H2O) and aqueous sodium hydroxide (NaOH). The exact composition of the reagent may vary depending on the source, but the basic components are copper (II) ions and hydroxide ions.
The copper ions in the reagent react with the peptide bonds in the protein, forming a complex that absorbs light at a specific wavelength. The hydroxide ions in the reagent help to maintain the pH of the solution and facilitate the formation of the complex.
The final solution is usually blue in colour, when protein is added to it, the formation of complex absorbs the blue light causing a shift in the colour of the solution from blue to green or blue-green, which indicates the presence of peptide bonds in the protein.
Procedure
- Take a small volume of test sample and place it in a test tube.
- Add a small amount of Biuret reagent the protein sample.
- Gently mix the solution.
- Observe the colour of the mixture. A change in colour, usually from blue to a shade of green or turquoise, indicates the presence of proteins in the sample.
- Compare the colour of the mixture to a standard colour chart or a sample known to contain a specific amount of protein to determine the approximate concentration of proteins in the original sample.
Specific Tests:
Xanthoproteic test
Principle
This test is used to identify the presence of aromatic amino acids (such as tryptophan and tyrosine) in a protein sample. The sample is treated with nitric acid, and the formation of a yellow colour indicates the presence of aromatic amino acids. The test is based on the fact that when tyrosine is heated with nitric acid, it undergoes a reaction that produces a yellow pigment called xanthoprotein.
Reagents
- Nitric acid (HNO3)
- 40 % NaOH
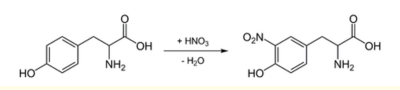
Fig: Xanthoproteic test
Procedure
- Take a small volume of test sample and place it in a test tube.
- Add a 1ml of nitric acid (HNO3) to the protein sample and mix well.
- Heat the mixture on a steam bath or in a hot water bath and cool the solution under tap water.
- Gently, add 2ml of 40 % NaOH to test tube until the mixture becomes alkaline and a colour change is noted
- Observe the colour of the mixture. A yellow colour indicates the presence of tyrosine in the protein sample.
- Compare the colour of the mixture to a standard colour chart or a sample known to contain tyrosine to determine the approximate concentration of tyrosine in the original sample.
Millon’s test
This test is used to identify the presence of tyrosine or phenylalanine in a protein sample.
Reagents
- Take a clean and dry glass container, add around 10-20 g of Mercuric acetate.
- Add approximately 10-20 ml of concentrated sulfuric acid, and 10-20 ml of Potassium hydroxide.
- Mix the solution properly.
- The reagent is now ready to use.
Procedure
- Take a small volume of test sample and place it in a test tube.
- Add a few drops of a reagent.
- Heat the mixture in a water bath at a temperature of around 80-90°C for 5-10 minutes.
- Observe the colour change of the solution, a positive result will show a pink to red colour indicating the presence of tyrosine in the sample.
- Compare the results with a known positive control sample.
Bradford test
It is a colorimetric assay for the determination of protein concentration. The Bradford reagent, which is composed of the dye Coomassie Brilliant Blue G-250 and a chemical stabilizer, binds to proteins through non-covalent interactions, and the intensity of the colour produced is directly proportional to the amount of protein present in the sample.
Reagents
Coomassie Brilliant Blue G-250 is a protein-staining dye that binds to the amino acid residues of the proteins. The binding of the dye to the protein causes a shift in the dye’s absorption spectra, resulting in a change in colour that is proportional to the amount of protein in the sample.
Dissolve 100 mg Coomassie Brilliant Blue G-250 in 50 ml 95% ethanol, add 100 ml 85% (w/v) phosphoric acid. When the dye has completely dissolved, dilute to 1 liter and filter through Whatman paper before using.
Procedure
- Prepare a standard protein solution with known concentrations of a protein, such as bovine serum albumin (BSA).
- Take a small amount of the sample to be tested (typically a few microliters) and place it in a cuvette or a test tube.
- Add a small amount of the Bradford reagent (Coomassie Brilliant Blue G-250) to the sample.
- Compare the color change of the sample to the color change of the standard protein solution, a positive result will show a similar color change indicating the presence of protein in the sample.
Sakaguchi Test
The Sakaguchi test is a biochemical test used to identify the presence of arginine in a sample. The test is based on the reaction of arginine with a reagent called Sakaguchi reagent, which is a mixture of nitrite and phenol. The reaction produces a red or pink color, indicating the presence of arginine in the sample. This test can be used in microbiology to identify different types of bacteria based on their arginine metabolism.
This test is used to detect the presence of arginine, specifically the presence of a guanido group. It results in a red colour change when positive.
Reagents
- 1 % α naphthol in alcohol
- Sodium hypochlorite
- 1 % urea solution
Procedure
- Prepare mixture of 2 ml test sample and 1 ml of 40%NaOH.
- Add 2-3 drops of α-naphthol. Mix it well and add 2-3 drop of sodium hypochlorite.
- Observe the colour of the mixture. A red or pink colour indicates the presence of arginine in the sample. The intensity of the colour is directly proportional to the concentration of arginine present in the sample.
- Compare the colour of the mixture to a control sample that does not contain arginine.
- Record the results and interpret them based on the colour of the mixture and the amount of arginine present in the sample.
Pfeiffer’s Test
Pfeiffer’s test, also known as the Pfeiffer’s reaction, is a qualitative test used to identify the presence of histidine in a sample. Histidine is an amino acid that contains a side chain of imidazole (-NH-CH=N-), which makes it different from other amino acids.
Histidine + Cu(II) → Histidine-Cu(II) complex (precipitate)
Reagents
Copper ions
Procedure
- Prepare mixture of 1 ml test sample and a few drops of solution of copper ions.
- The mixture is then heated, causing the imidazole side chain of histidine to react with the copper ions, producing a red-brown precipitate.
- The presence of a precipitate indicates a positive test for histidine.
Hopkins-Cole Test
The Hopkins-Cole test is a chemical test used to identify the presence of tryptophan in a protein sample. It is based on the reaction of tryptophan with pyridine and ninhydrin to produce a purple colour. In the presence of strong acid (H2SO4) indole group of tryptophan reacts with glyoxylic acid (Hopkins Cole reagent) to form violet colour ring
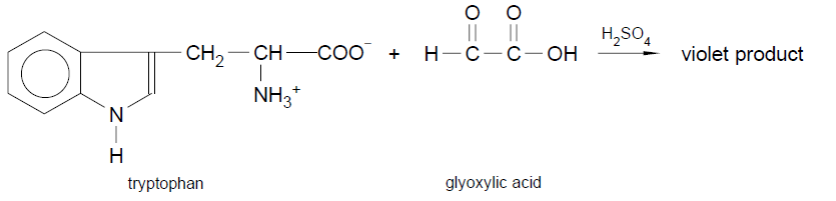
Fig: Hopkins-Cole Test
Reagents
- Mix equal volumes of 0.1M pyridine and 0.1% Ninhydrin to prepare Hopkins-Cole reagent.
- Add 2M Hydrochloric acid just before use to adjust the pH.
Procedure
- Prepare mixture of 2 ml test sample and 1 ml of 40%NaOH.
- Add 1 ml of Hopkins-cole reagent and mix gently.
- Gently, add few drops of H2SO4 slowly on the wall.
- Observe the colour of the mixture A purple violet ring will form
Note: All proteins will give positive result with Hopkin-Cole test except gelatin which contain little amount of tryptophan.
Nitroprusside test
The Nitroprusside test, also known as the Nitroprusside reduction test, is a laboratory test used to identify the presence of ketones in urine. This test uses sodium nitroprusside, a chemical reagent, which reacts with ketones to produce a colour change.
The nitroprusside test is specific for cysteine, the only amino acid with a sulfhydryl group (—SH). In an alkaline solution, the group interacts with nitroprusside to produce a red complex. Methionine does not respond to this test because it possesses a thioester bond that is difficult to break.
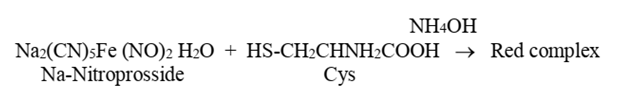
Fig: Nitroprusside test
Reagents
- Sodium nitroprusside (Freshly prepared 2%)
Sodium nitroprusside is typically provided as a solution in a dropper bottle. It is important to note that sodium nitroprusside is a light sensitive and should be stored in a dark place.
- Ammonium hydroxide
Procedure
- Prepare mixture of 2 ml test sample and 1 ml of 40%NaOH.
- Add 0.5 mL nitroprusside solution and mix thoroughly.
- Gently, add 0.5 mL ammonium hydroxide.
- Observe the colour of the mixture
- Formation of red colour will indicate the presence of cysteine in the test sample.
References:
- Bertholf Roger L. “Proteins and albumin”. Laboratory Medicine 45.1 (2014): e25-e41.
- Tiwari, A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- http://biocheminfo.com/
