Introduction:
The nitrogen cycle is a natural process in which nitrogen is transformed into various chemical forms as it passes through the environment. Nitrogen is important for life since it is a component of proteins, nucleic acids, and other chemical compounds that comprise living creatures.
Agriculture and the use of fossil fuels have both altered the nitrogen cycle’s natural balance. The use of nitrogen fertilizers in agriculture has resulted in an excess of nitrate in the soil, which can leak into groundwater and produce eutrophication in aquatic habitats. Eutrophication is the process through which excessive fertilizer inputs generate algal blooms, resulting in oxygen depletion and fish mortality.
The nitrogen cycle is critical for the survival of living creatures as well as the health of ecosystems. Understanding the nitrogen cycle is critical for minimizing the negative environmental effects of human activities such as eutrophication and greenhouse gas emissions. To maintain the equilibrium of the nitrogen cycle and preserve the sustainability of life on Earth, proper nitrogen management in agriculture, industry, and waste management is required.
Stages:
The nitrogen cycle is a natural process in which nitrogen is transformed into various chemical forms as it passes through the environment. The nitrogen cycle is divided into stages, each of which involves a unique set of species and activities. Each stage of the nitrogen cycle is interrelated and depends on the others to ensure the nitrogen balance in the environment. To maintain the equilibrium of the nitrogen cycle and preserve the sustainability of life on Earth, proper nitrogen management in agriculture, industry, and waste management is required.
The stages of the nitrogen cycle are as follows:
Nitrogen fixation
Nitrogen fixation is the process of converting atmospheric nitrogen gas (N2) into ammonia (NH3) or ammonium ions (NH4+) that living organisms can use. Nitrogen is a necessary element for the growth and development of living creatures; however, most organisms cannot directly use air nitrogen. As a result, nitrogen fixation is an essential process for the survival of life on Earth.
Nitrogen fixation can be classified into two types: biological and non-biological. Biological nitrogen fixation is accomplished by bacteria and archaea that may transform atmospheric nitrogen into ammonia or ammonium ions. These nitrogen-fixing bacteria can be found in soil, water, and the roots of some plants, such as legumes. Nitrogenase, an enzyme, is used by these bacteria to convert nitrogen gas into ammonia or ammonium ions.
Natural events such as lightning strikes cause non-biological nitrogen fixing. Lightning’s high temperature and pressure can cause air nitrogen to combine with oxygen to make nitrogen oxides (NOx), which can subsequently dissolve in rainwater and form nitric acid. This nitric acid can then be applied to the soil and utilized by plants.
Nitrification
Nitrification is the biological process by which ammonia (NH3) is converted into nitrite (NO2-) and then into nitrate (NO3-) by nitrifying bacteria. This process is important because it converts the ammonia produced during nitrogen fixation and ammonification into a form that can be taken up by plants.
Nitrification is a two-step process that requires oxygen and is carried out by two groups of bacteria: Nitrosomonas and Nitrobacter. Nitrosomonas bacteria convert ammonia into nitrite in the first step, and Nitrobacter bacteria convert nitrite into nitrate in the second step.
During the first step of nitrification, Nitrosomonas bacteria oxidize ammonia to nitrite in a reaction that releases energy and produces hydroxyl ions (OH-). The reaction is as follows:
NH3 + 1.5 O2 → NO2- + 2 H+ + H2O
During the second step of nitrification, Nitrobacter bacteria oxidize nitrite to nitrate in a reaction that also releases energy and produces hydroxyl ions. The reaction is as follows:
NO2- + 0.5 O2 → NO3-
The nitrate produced by nitrification is an important source of nitrogen for plants, as they can absorb it through their roots and use it to synthesize organic compounds, such as proteins and nucleic acids.
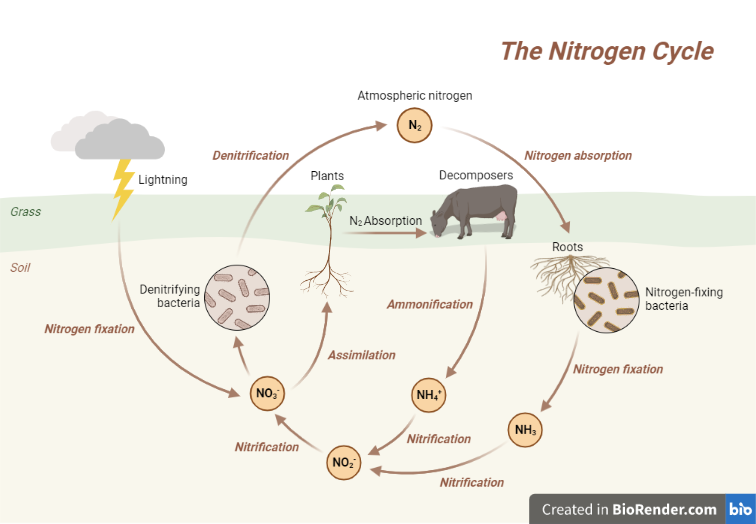
Fig: Nitrogen cycle
Assimilation
The process by which plants and animals incorporate nitrogen into their tissues in the form of organic substances such as amino acids, nucleotides, and proteins is known as assimilation. Assimilation is an important aspect of the nitrogen cycle because it allows nitrogen to be transferred from inorganic to organic form, making it useful by organisms.
Plant assimilation
Plants absorb inorganic nitrogen through their roots as nitrate (NO3-) or ammonium ions (NH4+). Nitrogen assimilation is the process by which inorganic nitrogen is transformed into organic nitrogen molecules once inside the plant. Plants use nitrogen in this process to produce amino acids, nucleotides, and proteins, all of which are required for plant growth and development.
Animal assimilation
Animals absorb organic nitrogen molecules by ingesting plants or other creatures that have already assimilated nitrogen. After that, the organic compounds are broken down into their constituent amino acids, which can then be used to make new proteins and other chemical compounds.
Plant and animal assimilation are both necessary for transferring nitrogen from its inorganic to organic form, allowing it to be used by species in the food chain.
Ammonification
It is a critical step in the nitrogen cycle, which converts nitrogen into various forms that can be utilized by living organisms. Organic nitrogen molecules, such as proteins and amino acids, are broken down into ammonia (NH3) by bacteria and fungi during ammonification. This ammonia is then used by other bacteria in the nitrogen cycle to generate nitrate (NO3-), which plants need as a nitrogen source. Ammonification is a crucial aspect of the nitrogen cycle because it recycles organic nitrogen molecules into a form that other species may utilize. Without ammonification, the nitrogen in organic matter would be inaccessible to other species, disrupting the nitrogen cycle.
Denitrification
It is a nitrogen cycle process in which bacteria convert nitrates (NO3-) into nitrogen gas (N2) or nitrous oxide (N2O), which is then released back into the atmosphere.
This happens in anaerobic conditions, such as waterlogged soils or sediments, where oxygen is unavailable for nitrifying bacteria to convert nitrates to nitrites (NO2-) and eventually to nitric oxide (NO) and nitrous oxide (N2O). Denitrifying bacteria, on the other hand, use nitrates as an alternate electron acceptor for respiration, converting them to nitrogen gas.
Importance:
Essential for the growth of plants:
Nitrogen is a vital component of proteins and nucleic acids, both of which are required for plant growth and development. The nitrogen cycle ensures that plants have a steady supply of nitrogen in the form of nitrates, which they can absorb via their roots.
Promotes biodiversity:
The nitrogen cycle supplies nitrogen to all living species, from microbes to plants to animals. This sustains a wide variety of life on Earth.
Maintains water quality:
The nitrogen cycle contributes to water quality by preventing the accumulation of excess nitrates, which can lead to eutrophication and other environmental issues.
Maintain the fertility of the soil:
The nitrogen cycle contributes to soil fertility by providing a continual supply of nutrients to plants. Organic nitrogen conversion into inorganic forms such as ammonia and nitrates also aid in the breakdown of organic matter and the release of nutrients back into the soil.
Management of the climate:
The nitrogen cycle plays a significant role in managing the Earth’s temperature by limiting the levels of greenhouse gases created during the denitrification process, such as nitrous oxide and methane.
Effects of human activities on nitrogen cycle:
Human activities have had a substantial impact on the nitrogen cycle, which has resulted in a variety of environmental issues. Changes to the nitrogen cycle caused by humans have far-reaching ecological consequences, affecting both natural ecosystems and human health. As a result, it is critical to reduce nitrogen pollution and implement sustainable measures to ensure the health of the ecosystem and its population.
| Human Activity | Effect on Nitrogen Cycle |
| Use of nitrogen fertilizers in agriculture | Increases available nitrogen for plant growth, but excessive use can lead to nitrate runoff and leaching, polluting water bodies and contributing to the formation of harmful algal blooms. |
| Burning of fossil fuels | Releases nitrogen oxides into the atmosphere, which can react with other pollutants to form acid rain, leading to soil acidification that affects the ability of plants to absorb nitrogen. |
| Livestock production | Generates large amounts of manure rich in nitrogen. If not managed properly, manure can release nitrogen into the environment, contributing to pollution. |
| Land use change (deforestation, urbanization) | Disrupts the nitrogen cycle, leading to soil erosion and loss of nitrogen-fixing trees, reducing the amount of nitrogen available for plant growth. |
| Industrial processes | Releases nitrogen compounds into the environment, contributing to air and water pollution. |
| Sewage treatment | Releases treated wastewater containing nitrogen compounds into the environment, contributing to nitrogen pollution. |
| Atmospheric deposition | The deposition of nitrogen from the atmosphere can increase the amount of nitrogen available for plant growth, but excessive deposition can lead to soil acidification and harm sensitive ecosystems. |
References:
- Galloway, J. N. et al. Year 2020: Consequences of population growth and development on deposition of oxidized nitrogen. Ambio 23, 120–123 (1994).
- Stein, L.Y. and Klotz, M.G., 2016. The nitrogen cycle. Current Biology, 26(3), pp.R94-R98.
- Howarth, R. W. Coastal nitrogen pollution: a review of sources and trends globally and regionally. Harmful Algae 8, 14–20. (2008).
