Introduction:
It is similar to compound microscopes except that their components are placed in inverted order. The specimen is viewed from below and the source of light and condenser are positioned above the specimen.
Light is directed down onto the stage/specimen in this design, and specimens in larger laboratory containers, such as flasks and Petri dishes, can be viewed accordingly. When an auxiliary microscope camera is used, specimens can be examined through the lens or on a screen.
Inverted microscope design extends the benefits of the light microscope that enable to observe micro-organisms in a large container under more natural conditions.
Inverted microscopes are best suited for the large specimens such as tissue cultures, precipitates, sediment and reactions. Likewise, the sample life can be extended because the specimen is eventually protected from the light.
Inverted microscopes are used to observe cells and organisms in culture, such as Petri dishes, flasks, microplates, and roller bottles. They have brightfield and frequently phase contrast, allowing them to see unstained translucent tissue more clearly. Fluorescence observation can also be added for a variety of fluorescence dyes.
Background:
Lawrence Smit of Tulane University and colleagues constructed the first inverted microscope for examining biological material in 1850. This innovation resulted in large-scale production and widespread acceptance in the scientific community. The inverted microscope, also known as a cell culture or tissue culture microscope, provides higher magnification from 4x to 40x.
Equipped with three to six objective lenses and a condenser lens, it facilitates live-cell imaging, making it a preferred tool for researchers. Its advantages over upright microscopes underscore its enduring significance in scientific research.
Principle:
The principle of an inverted microscope is identical to that of standard microscopy, with the emphasis on magnification. The inverted microscope, on the other hand, has the condenser lens and light source located at the top. Images are created by reflecting light through the condenser lens and using the diffraction mechanism for magnification. The objective lenses below the stage of an inverted microscope, typically three to six, mediate light beams, allowing detailed observation of biological objects in culture containers. When studying live cells and tissues, high optical capacity vessels and high-quality coverslips are critical for optimal picture generation.
Parts:
Base: It provides stability and support.
Illumination Source: It guides light from above through the specimen.
Condenser: It concentrate and controls light for better visibility.
Objective Lenses: Positioned beneath the specimen for magnification.
Stage: The specimen is placed which is designed for various containers.
Focus Adjustment Mechanism: It allows coarse and fine focusing.
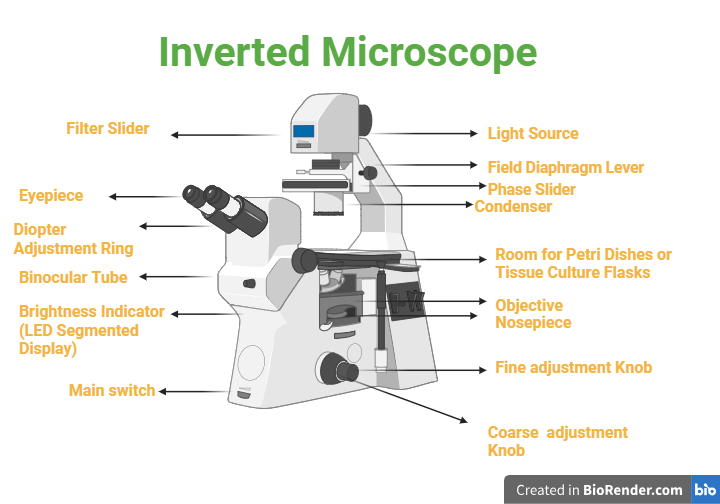
Fig: Parts of Inverted Microscope
Nosepiece: It hold numerous objective lenses.
Eyepieces: Lenses for observing the magnified specimen.
Tube or Body: Connects eyepieces to objective lenses.
Fine and Coarse Focus Controls: It empower precise focus adjustment.
Mechanical Stage Controls: It allow precise movement for scanning.
Filter Cube Holder (optional): It contain fluorescence filters.
Phase Contrast or DIC Components (optional): Enhance contrast in translucent specimens.
Applications:
- Transmitted and reflected light in the inverted microscope is generally used for the study the cultured organization and sediment in culture bottles and culture dishes
- This device can be used in bright field, dark field, phase contrast, differential interference contrast, fluorescence observation, and polarized light.
- It is employed in the study of human blood and tissue samples, observation of the connection, activity and growth between live cells. minimally invasive, external fertilization, toxicity test, drug reaction, microscopic observation drug sensitivity (MODS) assay, etc.
Care and maintenance:
Lenses and filters
- It is highly recommended to clean lens surfaces or filters. Firstly, remove dust using compressed air. In case, if dust still persists, better use a soft/clean brush or gauze.
- In order to remove grease or fingerprints it is suggested to used a soft gauze or lens tissue that can also be gently moistened using pure alcohol.
- Petroleum benzine is applied to clean the immersion oil from objective lenses. However, it is always suggested to be careful handing petroleum benzine because it is highly flammable.
Cleaning of painted or plastic components
• Using of organic solvents such as alcohol, ether, etc. is highly restricted discouraged. Using of such solvents could result in discoloration or in the peeling of paint.
When not in use
- It is highly recommended to cover the instrument with dust cover and store in a place with low humidity (maximum humidity of 85%) where chance of formation of mould is minimum.
- Always store the eyepieces, objectives, and filters in a container or desiccator with drying agent.
- Turn off and unplug the microscope before moving.
- Microscope should be kept at temperatures between 0°C-40°C
- The quality of the specimen imaging is highly influenced by the environment. So, don’t placed the microscope in direct sunlight or under direct indoor light.
