Introduction to Herpesviruses:
The herpesvirus family contains several of the most important human pathogens. Clinically, the herpesviruses exhibit a spectrum of diseases.
The herpesviruses that commonly infect humans include herpes simplex virus types 1 and 2,
varicella-zoster virus, cytomegalovirus, Epstein-Barr virus, herpesviruses 6 and 7, and
herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus).
Classification of Human Herpesviruses:
Classification of the numerous members of the herpesvirus family into subfamilies is based on biologic properties of the agents and includes
Alpha herpesviruses
Alphaherpesviruses are fast-growing, cytolytic viruses that tend to establish latent infections in neurons; herpes simplex virus and varicella-zoster virus are members.
Betaherpesvirus
Betaherpesviruses are slow-growing and may be cytomegalic (massive enlargements of infected cells) and become latent in secretory glands and kidneys; member includes cytomegalovirus
Gammaherpesvirus
Gammaherpesviruses includes Epstein-Barr virus (genus Lymphocryptovirus) that infect and become latent in lymphoid cells.
Replication Cycle of Herpesvirus:
Virus Entry & Attachment
- Herpesviruses enter host cells by fusing with the cell membrane.
- Entry begins when viral envelope glycoproteins bind to specific cell surface receptors, mainly heparan sulfate.
- Attachment also requires interaction with co-receptors, often from the immunoglobulin superfamily.
Capsid Transport & DNA Delivery
- After fusion, the viral capsid travels through the cytoplasm to a nuclear pore.
- Uncoating occurs at the nuclear pore, releasing viral DNA into the nucleus.
- The viral DNA immediately circularizes upon entry into the nucleus.
Regulation of Gene Expression
- Herpesvirus gene expression follows a sequential cascade: immediate-early (α), early (β), and late (γ) phases.
- VP16, a viral tegument protein, activates transcription of immediate-early genes.
- Alpha (α) proteins produced from these genes enable transcription of early (β) genes.
- Beta (β) proteins include enzymes and DNA-binding proteins essential for viral DNA replication.
- Once DNA replication begins, late (γ) genes are expressed, producing structural proteins.
Viral Genome Replication & Protein Synthesis
- Viral DNA is replicated using a rolling-circle mechanism.
- Transcription of viral genes is carried out by host RNA polymerase II, with the help of viral factors.
- In infected cells, over 50 different viral proteins are synthesized.
- Alpha and beta proteins mainly regulate replication and gene expression.
- Gamma proteins are primarily involved in forming new virus particles.
Assembly & Maturation
- Newly made viral DNA is packaged into pre-formed capsids within the nucleus.
- Viral particles mature by budding through the modified inner nuclear membrane, acquiring their envelope.
- Enveloped virions are transported via vesicles to the cell surface for release.
Replication timing and effects on host cell
- Replication cycle length varies
- ~18 hours for Herpes Simplex Virus (HSV)
- Over 70 hours for Cytomegalovirus (CMV)
- Infected cells are eventually destroyed (cytolysis).
- Host macromolecule synthesis is shut down early in infection.
- Cellular DNA and protein production stop as viral activity takes over.
Genomic Complexity:
- Herpesvirus genomes contain 70 to over 200 open reading frames (ORFs).
- These ORFs encode a wide array of regulatory, enzymatic, and structural proteins.
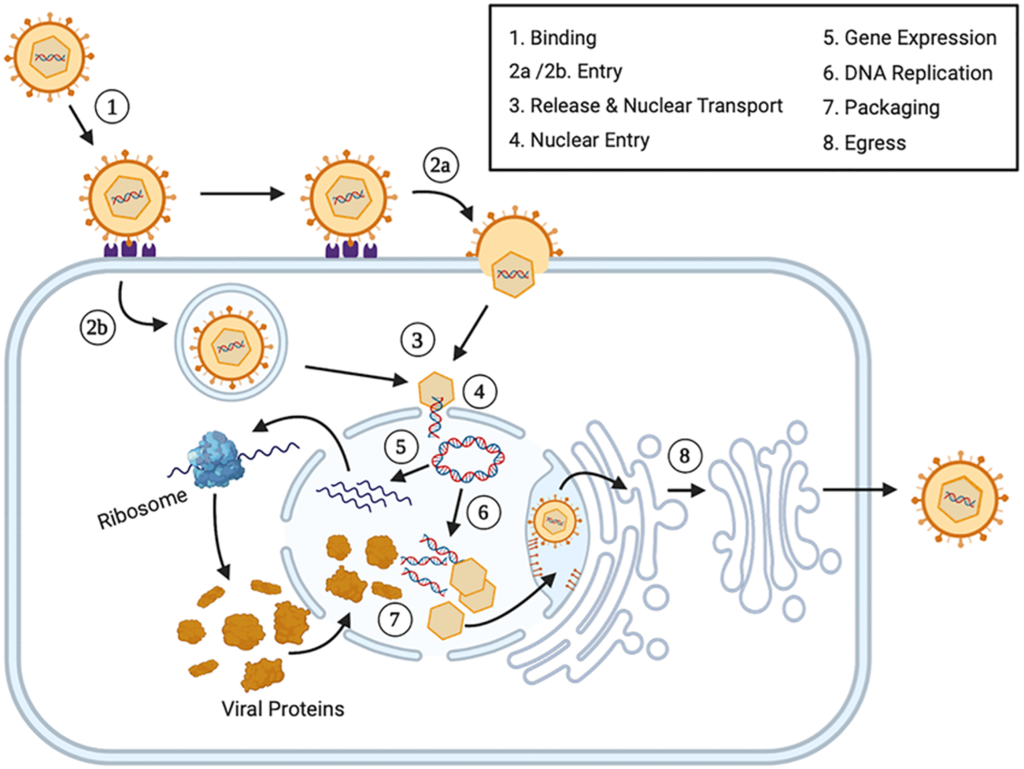
Fig: Simple representation of replication cycle of Herpes virus
Overview of Major Human Herpesvirus Diseases:
- Herpes Simplex Virus Type 1 (HSV-1)
- Herpes Simplex Virus Type 2 (HSV-2)
- Varicella-Zoster Virus (VZV)
- Cytomegalovirus (CMV)
- Epstein-Barr Virus (EBV)
- Human Herpesvirus 6 (HHV-6)
- Human Herpesvirus 7 (HHV-7)
- Human Herpesvirus 8 (HHV-8 / Kaposi’s Sarcoma-Associated Herpesvirus)
Type 1 and 2 Herpes simplex virus cause latent infections in neurons and infect epithelial cells. Type 1 is frequently linked to oropharyngeal lesions and frequently results in “fever blisters.”
Type 2 herpesvirus is the primary cause of genital herpes, a sexually transmitted infection that causes recurrent outbreaks of sores or blisters in the genital area.
The cytomegalovirus stays in lymphocytes and replicates in the epithelial cells of the kidneys, salivary glands, and respiratory system. An infectious mononucleosis is the result. Cytomegalic inclusion disease can happen to newborns. A significant contributor to mental retardation and congenital abnormalities is cytomegalovirus.
T cells are infected by the human herpes virus 6. It causes exanthem subitum (roseola infantum) and is usually acquired in early infancy. Exanthem subitum, sixth disease, pseudorubella, exanthem criticum, and three-day fever are other names for Roseola infantum, a clinical phenomenon that is marked by a high temperature (which can reach 104ºF) that lasts for three to five days before suddenly going away and developing a rash.
A T-lymphotropic virus, humanherpesvirus7, has not yet been connected to any particular illness.
The Epstein-Barr virus creates latent infections in lymphocytes and replicates in the parotid gland and oropharyngeal epithelial cells. It is the cause of human lymphoproliferative diseases, particularly in immunocompromised patients, and infectious mononucleosis.
Human herpesvirus 8 appears to be associated with the development of Kaposi’s sarcoma, a vascular tumor that is common in patients with AIDS.
Human herpesviruses are frequently reactivated in immunosuppressed patients (transplant recipients, cancer patients etc) and may cause severe disease, such as pneumonia or lymphomas. Herpesviruses have been linked with malignant diseases in humans.
