Introduction:
Heat-shock proteins (HSPs) are a member of a large protein family that is widely expressed in the majority of species in response to unfavorable condition such as heat and other forms of cellular stress. However, they are not simply expressed throughout the normal growth process of cell cycle. The main purpose of HSPs is to protect cells from heat and other forms of cellular stress by acting as chaperones for protein folding and trafficking, maintaining physiological function. Moreover, in response to a range of diverse stress stimuli, such as heavy metals, inflammatory cytokines, amino acid analogues, oxidative stress, or ischemia, all cells and species from bacteria to humans express HSPs.
History:
Heat shock proteins (HSPs) are a family of proteins that were first discovered in the late 1960s by a scientist named Ferruccio Ritossa. Ritossa was studying the phenomenon of chromosome puffing in the fruit fly Drosophila melanogaster, which occurs when certain regions of the chromosome become more active and therefore appear swollen or “puffed” under a microscope.
During his experiments, Ritossa noticed that when he exposed the flies to high temperatures, certain regions of the chromosomes became even more puffed up than usual. He realized that this was due to the production of a new set of proteins that were being produced in response to the heat stress.
These proteins were later named heat shock proteins because they are synthesized in response to stressors such as high temperatures, toxins, and other environmental stresses that can cause damage to cells. The proteins act as molecular chaperones, helping to fold other proteins into their correct three-dimensional structures and prevent damage to cells.
Regulation:
HSPs are controlled by a complex network of signaling pathways that respond to numerous stressors and cellular stimuli. The activation of heat shock factor (HSF) transcription factors, which activate the expression of HSP genes in response to stress, is the primary regulator of HSP expression and activity. After HSPs are generated, their activity can be controlled further through post-transcriptional mechanisms including as phosphorylation, acetylation, and ubiquitination, as well as proteasomal destruction. HSPs can also control their own expression via feedback inhibition. Overall, HSP regulation is a dynamic process involving several levels of control that allows cells to fine-tune their response to stress and maintain protein homeostasis under varying situations.
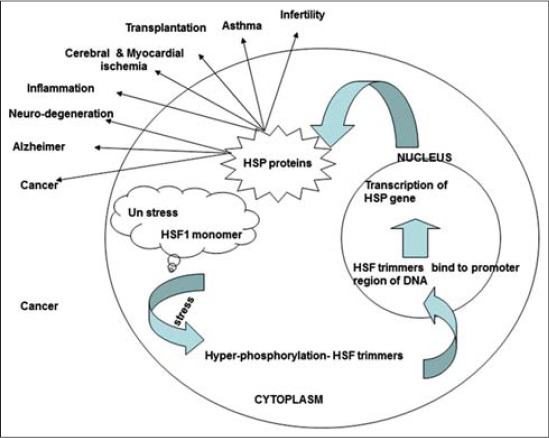
Source: https://doi.org/10.14202/vetworld.2015.46-51
Fig: Regulation and role of heat shock proteins.
Heat shock response (HSR):
The heat shock response (HSR) is a cellular stress response mechanism that is triggered when cells are subjected to high temperatures or other types of stress such as oxidative stress, toxins, or radiation. The HSR is a highly conserved reaction found in all forms of life, including microbes and humans.
When cells are stressed, the HSR is triggered by the activation of a group of transcription factors known as heat shock factors (HSFs). HSFs are activated by a number of cellular stressors and then attach to heat shock elements (HSEs) in the DNA to initiate the expression of heat shock proteins (HSPs).
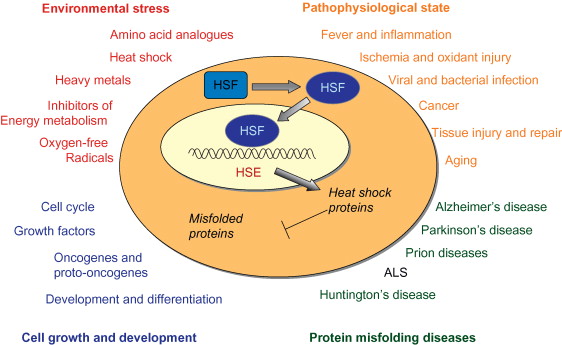
Source: https://doi.org/10.1016/B978-0-12-374145-5.00268-0
Fig: Conditions that activate the heat shock response
Types and their Functions:
There are several types of heat shock proteins (HSPs) with distinct functions, classified based on their molecular weight and sequence homology. Here are some of the most important HSPs and their functions:
Hsp60
This is a member of the HSP (heat shock protein) family that functions in the cell as a molecular chaperone. It is found in the mitochondria and is essential for protein folding and assembly in the mitochondrial matrix. It can also be found in other cellular compartments like the cytosol and the endoplasmic reticulum.
It helps in folding of newly generated proteins, ensuring that they are correctly folded and functioning by attaching to hydrophobic regions on unfolded proteins, preventing non-functional aggregates from forming.
HSP70
It is found in every cell compartment, including the cytosol, nucleus, endoplasmic reticulum, and mitochondria. It helps to prevent protein aggregation and misfolding in response to cellular stress, such as heat shock, oxidative stress, or toxin exposure by attaching to the exposed hydrophobic areas of partially unfolded or denatured proteins, preventing them from forming non-functional aggregates or degrading.
HSP90
It is found in all cellular compartments, including the cytosol, nucleus, endoplasmic reticulum, and mitochondria.
The primary function of HSP90 is to assist in the folding, stabilization, and activation of a wide range of client proteins, which play key roles in cellular signaling pathways, such as kinases and transcription factors. HSP90 is also involved in the regulation of protein degradation through interactions with ubiquitin ligases and the proteasome.
HSP90 is highly expressed in cancer cells and has been identified as a potential target for cancer therapy. Inhibition of HSP90 can lead to the degradation of its client proteins, including those that drive cancer cell proliferation and survival, making it a promising target for cancer treatment.
HSP110
Hsp110 is a large HSP found in the cytoplasm that plays a key role in protein folding and degradation pathways. It is particularly important in response to heat shock and other forms of stress, where it helps to maintain cellular homeostasis and prevent damage to cellular structures. In addition, HSP110 has been implicated in a variety of cellular processes, including cell division, protein trafficking, and signal transduction.
Small HSPs
Small HSPs (sHSPs) are a class of heat shock proteins distinguished by their low molecular weight (varying from 12-43 kDa). They can be present in the cytosol, nucleus, endoplasmic reticulum, and mitochondria, among other places. The primary role is this HSPs is to act as molecular chaperones, preventing protein aggregation and misfolding in response to stress, such as heat shock, oxidative stress, or toxin exposure. sHSPs bind to partially unfolded or denatured proteins, preventing them from aggregating and aiding refolding.
Functions:
They play a critical role in cellular homeostasis by regulating protein folding, assembly, and degradation. HSPs are induced by a variety of environmental and cellular stresses, such as heat, toxins, oxidative stress, and inflammation.
Biological Functions
One of the primary functions of HSPs is to act as molecular chaperones, which facilitate the proper folding and assembly of newly synthesized proteins, preventing their aggregation and degradation. Additionally, HSPs are involved in regulating protein degradation pathways, maintaining cellular energy balance, and assisting in protein trafficking and signaling.
Pathological Roles
Abnormal regulation of HSPs has been implicated in several pathological conditions, including cancer, neurodegenerative disorders, and autoimmune diseases. In cancer, HSPs can promote tumor growth by preventing cell death and aiding in the development of resistance to chemotherapy. In neurodegenerative disorders, HSPs may play a protective role by facilitating the clearance of misfolded proteins, but excessive HSP expression has also been linked to disease progression. In autoimmune diseases, HSPs can trigger an immune response by serving as autoantigens.
Therapeutic Opportunities
Given the crucial roles of HSPs in cellular homeostasis and their involvement in various diseases, HSPs have emerged as promising therapeutic targets. One approach involves the use of small molecules to modulate HSP expression or activity, with the goal of enhancing or reducing HSP function in different contexts. Other strategies aim to exploit HSPs as vaccine targets or utilize them as carriers for delivering drugs to specific tissues or cells. Additionally, the use of HSP inhibitors in cancer treatment has shown promise in preclinical studies.
Biomedical applications
HSPs have been revealed to have various biological applications in recent years, including cancer therapy, neurological disorders, wound healing, and vaccine development. Many cancer cells have high levels of HSPs, which protect them against the adverse effects of chemotherapy or radiation therapy. Researchers are looking for ways to limit HSP activity in order to render cancer cells more vulnerable to therapy. HSPs can also stimulate wound healing and tissue regeneration while protecting brain cells from neurodegenerative disorders. HSPs can also be exploited as antigen carriers in vaccine development. HSPs’ potential medical applications are still being investigated, although they represent a promising field of study for future biological applications.
References:
- Dubey A, Prajapati KS, Swamy M, Pachauri V. Heat shock proteins: a therapeutic target worth to consider. Vet World. 2015 Jan;8(1):46-51.
- Morimoto, R.I. and Westerheide, S.D., 2010. The heat shock response and the stress of misfolded proteins. In Handbook of cell signaling (pp. 2231-2239). Academic Press.
- Hu C, Yang J, Qi Z, Wu H, Wang B, Zou F, Mei H, Liu J, Wang W, Liu Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm (2020). 2022 Aug 2;3(3):e161
- Miller, D.J. and Fort, P.E., 2018. Heat shock proteins regulatory role in neurodevelopment. Frontiers in neuroscience, 12, p.821.
- Binder RJ. Heat-shock protein-based vaccines for cancer and infectious disease. Expert Rev Vaccines. 2008 Apr;7(3):383-93.
