Introduction:
Glycogenolysis is the biochemical pathway in which chain of glycogen molecule is breaks down into glucose monomers to provide immediate energy or to maintain blood glucose levels during times of need.
Glycogen is the principal storage form of glucose. Blood glucose is a source of energy for the entire human body. During the fasting state, to maintain normal blood glucose levels, the liver plays a central role in producing glucose through this catabolic process.
Location:
It occurs in the cytoplasm of the cells in the adipose, liver, and muscle tissues.
Requirements:
Three enzymes function to transport the glucose molecules from the outer branches of glycogen into the glycolytic pathway. They are:
Glycogen phosphorylase
It catalyses the reaction in which a linkage between two glucose residues at a nonreducing end of glycogen undergoes attack by inorganic phosphate. Pyridoxal phosphate is an essential cofactor in this reaction.
Glycogen debranching enzyme
It breaks α-1,6 linkages to release single units of glucose-1- phosphate.
Phosphoglucomutase
It converts glucose- 1-phosphate to glucose-6-phosphate, which is then connected into the glycolytic pathway.
Steps:
Glycogen phosphorolysis
Glycogen degradation is initiated by the action of phosphorylase which catalyses the rupture of α1→4 glycosidic bonds by insertion of a phosphate at carbon 1 on the nonreducing ends of glycogen branches, yielding glucose-1-phosphate.
Until it reaches a position four glucose residues from a (α 1-6) branch point, glycogen phosphorylase repeatedly works on the nonreducing ends of glycogen branches.
It results in a form of glycogen known as limit dextrin that cannot be further degraded by glycogen phosphorylase.

Fig: Removal of a terminal glucose residue from the nonreducing end of a glycogen chain by glycogen phosphorylase

Fig: Glycogen degradation to glucose
Hydrolysis of α1→6 glycosidic bonds
This is catalysed by α1→6-glucosidase, or debranching enzyme, which releases free glucose.
Following the action of debranching enzyme, phosphorylase keeps releasing glucose-1-phosphate until it is four glucose molecules away from the subsequent next α1→6 bond.
Formation of Gucose-6-Phosphate
Through the combined action of glycogen phosphorylase and debranching glucose-l-phosphate is converted into glucose-6-phosphate by the enzyme phosphoglucomutase. This is the reverse process that occurs during glycogenesis to produce glucose-1-phosphate.
Free glucose formation
The last stage of glycogenolysis is the hydrolysis of gluoce-6-Phosphate to glucose and inorganic phosphate, catalysed by gluocose-6-Phosphatase.
The membranes of the liver and kidneys, as well as the endoplasmic reticulum of intestinal cells, all contain this enzyme, whereas muscle cells do not. This reveals that muscle cannot transfer glucose into the bloodstream but the liver, kidney, and intestine can.
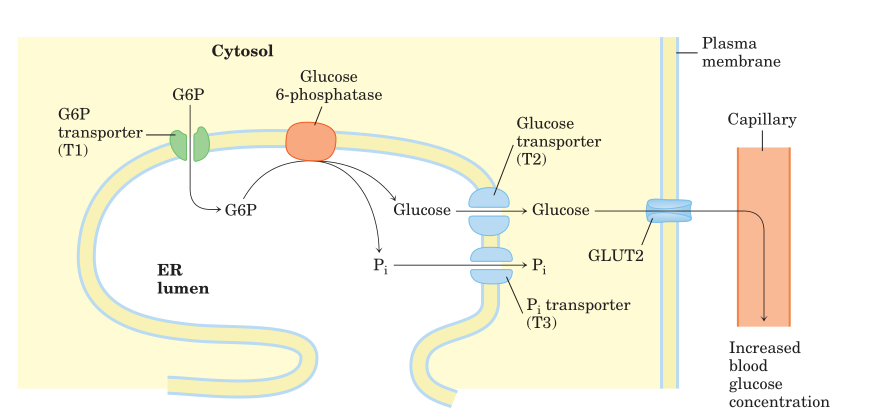
Fig: Hydrolysis of glucose 6-phosphate by glucose 6-phosphatase
Regulations:
A good coordination and regulation of glycogen synthesis and its degradation are essential to maintain the blood glucose levels.
Allosteric regulation
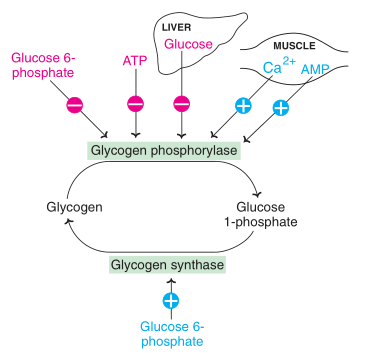
Fig: Allosteric regulation of glycogenolysis and glycogenesis (-: Inhibition; +: activation)
Hormonal Regulation
Glucagon, insulin, and epinephrine are the hormones that control and enhance glycogenolysis in response to blood sugar levels.
Clinical aspects:
- Several human genetic disorders that are caused by mutations in the relevant enzymes involved in the pathway emphasize the significance of glycogen metabolism.
- The liver, muscle, heart, kidney, and/or brain all usually show some degree of malfunction as a result of abnormalities in glycogen metabolism. Furthermore, depending on the enzyme affected, the observed symptom spectrum seems to be quite variable.
- It has been reported that an accumulation of inclusion bodies in several tissues contain insoluble glycogen and resistant to degradation. Therefore, its accumulation turns toxic to neurons, resulting in cell death.
- An enzyme deficiency in the body inhibits the storage or proper breakdown of the glycogen, which result in a genetic condition known as Glycogen Storage Disease (GSD) that primarily affects liver, muscles and other parts of the body.
References:
- Ellingwood SS, Cheng A. Biochemical and clinical aspects of glycogen storage diseases. J Endocrinol. 2018 Sep;238(3):R131-R141.
- David, L., Nelson, D.L., Cox, M.M., Stiedemann, L., McGlynn Jr, M.E. and Fay, M.R., 2000. Lehninger principles of biochemistry.
- Satyanarayana, U., 2021. Biochemistry, 6e-E-book. Elsevier Health Sciences.
- Blanco, A. and Blanco, G., 2017. Carbohydrate metabolism. Medical Biochemistry, 1261, pp.283-323.
- Paredes-Flores, M.A. and Mohiuddin, S.S., 2021. Biochemistry, Glycogenolysis. In StatPearls [Internet]. StatPearls Publishing.
