Introduction:
- Gene therapy is a type of medical treatment that involves the use of genetic material to treat or prevent diseases. It is based on the idea of replacing or modifying faulty genes or adding new genes to an individual’s genome in order to correct genetic abnormalities or to enhance the body’s natural abilities to fight diseases.
- Gene therapy can be used to treat a wide range of genetic disorders and diseases, including cancer, inherited genetic disorders, and infectious diseases. It can be administered in several different ways, including through the use of viral vectors, which are modified viruses that can deliver healthy genetic material to cells in the body, or through the use of non-viral vectors, which can deliver genetic material to cells without the use of a virus.
- Gene therapy is still a relatively new field, and while it has shown great promise in early research and clinical trials, it is not yet widely available as a treatment option. However, scientists and medical researchers are continuing to work on developing and refining gene therapy techniques and therapies, and it is expected that gene therapy will play an increasingly important role in the treatment of genetic disorders and diseases in the future.
Classification:
Gene therapy can be classified in several different ways.
Type of genetic material used
Gene therapy can use different types of genetic material, including DNA, RNA, or gene editing tools such as CRISPR/Cas9.
Target cells
Gene therapy can target different types of cells, including germline cells (reproductive cells), somatic cells (non-reproductive cells), cancer cells, or immune cells.
Vector used
Gene therapy can use different types of vectors to deliver the genetic material to cells, including viral vectors (modified viruses) or non-viral vectors (such as liposomes or nanoparticles).
Mode of delivery
Gene therapy can be delivered to cells in different ways, including intravenous injection, intratumoral injection, topical application, inhalation, or gene gun.
Type of genetic disorder or disease being treated
Gene therapy can be used to treat a wide range of genetic disorders and diseases, including inherited genetic disorders, cancer, infectious diseases, cardiovascular disease, and neurological disorders.
Form of gene therapy
Gene therapy can be classified as germline gene therapy (modifying the genes of reproductive cells), somatic gene therapy (modifying the genes of non-reproductive cells), autologous gene therapy (using an individual’s own cells), allogeneic gene therapy (using cells from a donor), or ex vivo gene therapy (modifying cells outside of the body).
Methods:
- Intravenous injection: This involves injecting the gene therapy directly into the bloodstream, where it can be taken up by cells throughout the body.
- Intramuscular injection: This involves injecting the gene therapy directly into a muscle, where it can be taken up by muscle cells.
- Intratumoral injection: This involves injecting the gene therapy directly into a tumor, where it can be taken up by cancerous cells.
- Topical application: This involves applying the gene therapy directly to the skin or mucous membranes, where it can be taken up by cells in the affected area.
- Inhalation: This involves inhaling the gene therapy, which can be taken up by cells in the respiratory system.
- Gene gun: This involves using a device to deliver the gene therapy directly to cells in the skin or other tissues.
Techniques:
The technique that is used for gene therapy will depend on the specific type of genetic disorder or disease being treated and the characteristics of the individual’s cells.
Viral vectors
Viral vectors are modified viruses that can deliver healthy genetic material to cells in the body. They are the most common method of delivery for gene therapy. Some common types of viral vectors include adenoviruses, adeno-associated viruses (AAVs), and retroviruses.
Non-viral vectors
Non-viral vectors can also be used to deliver genetic material to cells without the use of a virus. Some common types of non-viral vectors include liposomes, nanoparticles, and cationic polymers.
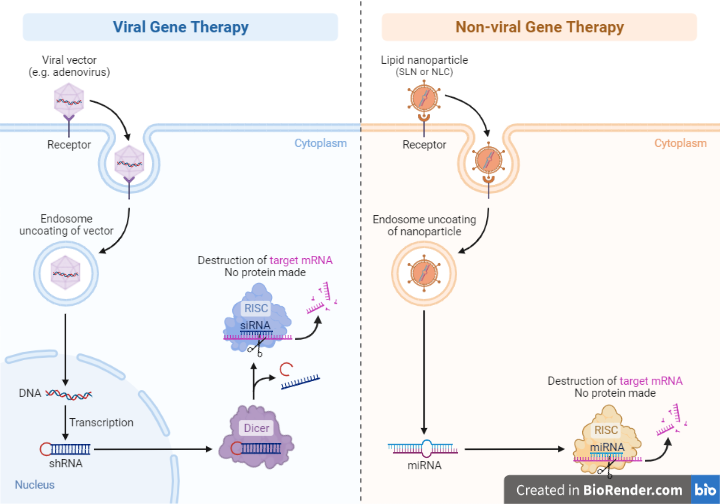
Fig: Viral vs Non-viral Gene Therapy
Gene editing tools
Gene editing tools, such as CRISPR/Cas9, TALENs, and zinc finger nucleases, can be used to make precise changes to an individual’s genome in order to correct genetic defects or to alter the expression of certain genes.
Ex vivo gene therapy
Ex vivo gene therapy involves modifying cells outside of the body and then transplanting the modified cells back into the individual’s body.
Procedure:
Identifying the target gene: The first step in gene therapy is to identify the specific gene or genes that are responsible for the genetic disorder or disease being treated. This can be done using various techniques, such as genetic testing or genomic sequencing.
Developing the gene therapy vector: Once the target gene has been identified, the next step is to develop a gene therapy vector, which is a vehicle that will be used to deliver the functional copy of the gene to the target cells. There are several types of gene therapy vectors, including viral vectors (such as adenoviruses, lentiviruses, or adeno-associated viruses) and non-viral vectors (such as liposomes or nanoparticles).
Preparing the gene therapy vector: The gene therapy vector is then prepared by inserting the functional copy of the target gene into the vector, ensuring that it is in the correct orientation and under the control of the appropriate regulatory elements.
Delivering the gene therapy vector: The gene therapy vector is then delivered to the target cells, which can be achieved through various methods, such as intravenous injection, intracellular injection, or delivery using nanoparticles or viral vectors.
Targeting the gene therapy vector: Once inside the cell, the gene therapy vector must be targeted to the appropriate location in the genome in order to ensure that the functional copy of the target gene is properly expressed. This can be achieved through the use of specific regulatory elements or by using targeted delivery methods such as CRISPR/Cas9.
Expressing the target gene: Once the functional copy of the target gene has been delivered and targeted to the appropriate location in the genome, it is expressed, resulting in the production of the protein encoded by the gene. This can help to correct the genetic disorder or treat the disease.
Applications:
Gene therapy is still a relatively new field, and while it has shown great promise in early research and clinical trials, it is not yet widely available as a treatment option. However, scientists and medical researchers are continuing to work on developing and refining gene therapy techniques and therapies, and it is expected that gene therapy will play an increasingly important role in the treatment of genetic disorders and diseases in the future.
Inherited genetic disorders: Gene therapy can be used to treat inherited genetic disorders, such as cystic fibrosis, sickle cell anemia, and thalassemia, by replacing or modifying the faulty genes that cause these conditions.
Cancer: Gene therapy can be used to treat cancer by adding genes that can help to kill cancer cells or by suppressing the expression of cancerous genes.

Fig: Cancer Cell-Targeted Gene Therapy
Infectious diseases: Gene therapy can be used to treat infectious diseases by adding genes that can help to boost the body’s natural immune response to the infection.
Cardiovascular disease: Gene therapy can be used to treat cardiovascular diseases, such as heart failure and coronary artery disease, by adding genes that can help to improve heart function or by suppressing the expression of genes that contribute to the development of these conditions.
Neurological disorders: Gene therapy can be used to treat neurological disorders, such as Parkinson’s disease and Alzheimer’s disease, by adding genes that can help to protect nerve cells or by suppressing the expression of genes that contribute to the development of these conditions.
Gene Therapy Treatment Phases:
Gene therapy treatments can take many years to develop and progress through the different phases of testing. It is important to carefully evaluate the safety and effectiveness of gene therapy treatments before they are made widely available to the public.
Preclinical research: This phase involves conducting laboratory and animal studies to assess the safety and effectiveness of the gene therapy treatment. This phase is necessary to determine whether the treatment is ready for testing in humans.
Phase I clinical trial: This phase involves testing the gene therapy treatment in a small group of people (typically between 10 and 30 individuals) to assess its safety and to determine the appropriate dosage.
Phase II clinical trial: This phase involves testing the gene therapy treatment in a larger group of people (typically between 30 and 100 individuals) to assess its effectiveness and to further evaluate its safety.
Phase III clinical trial: This phase involves testing the gene therapy treatment in an even larger group of people (typically hundreds or thousands of individuals) to confirm its effectiveness, monitor its side effects, and compare it to other treatments.
Regulatory approval: If the gene therapy treatment is shown to be safe and effective in clinical trials, it may be approved for use by regulatory agencies, such as the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA).

Post-market surveillance: After the gene therapy treatment has been approved and is being used in clinical practice, it is important to continue monitoring its safety and effectiveness through post-market surveillance. This may involve collecting data from patients who have received the treatment and analyzing the results to identify any potential issues or adverse effects.
Limitations:
Gene therapy is a promising field of medicine that has the potential to treat a wide range of genetic disorders and diseases. However, it is still a relatively new field, and there are several limitations and challenges that need to be overcome in order to make it more widely available and effective as a treatment option.
Delivery of genetic material: One of the major challenges of gene therapy is finding an effective and safe way to deliver the genetic material to the targeted cells. Viral vectors, which are modified viruses that can deliver healthy genetic material to cells in the body, are often used for this purpose. However, there is a risk of immune reactions or other adverse effects when using viral vectors. Non-viral vectors, which can deliver genetic material to cells without the use of a virus, are also being developed, but they are less efficient at delivering genetic material to cells.
Gene expression and regulation: Another challenge of gene therapy is ensuring that the genetic material is expressed and regulated in a controlled and sustained manner. In some cases, the genetic material may not be expressed at all, or it may be expressed in an uncontrolled manner, leading to unintended consequences.
Immune reactions: There is a risk of immune reactions when using gene therapy, particularly when using viral vectors. The body’s immune system may recognize the viral vector as a foreign invader and attack it, leading to an immune reaction.
Ethical concerns: There are also ethical concerns surrounding the use of gene therapy, particularly with regards to germline gene therapy, which involves modifying the genes of reproductive cells. There is concern that such modifications could have unintended consequences and could be used to create “designer babies” or to enhance certain traits in individuals.
Cost: Gene therapy can be expensive, and it is not yet widely available as a treatment option. This can make it difficult for some individuals to access this type of treatment.
References:
- Wirth, T., Parker, N. and Ylä-Herttuala, S., 2013. History of gene therapy. Gene, 525(2), pp.162-169.
- Dunbar, C.E., High, K.A., Joung, J.K., Kohn, D.B., Ozawa, K. and Sadelain, M., 2018. Gene therapy comes of age. Science, 359(6372), p.eaan4672.
- Verma, I.M. and Weitzman, M.D., 2005. Gene therapy: twenty-first century medicine. Annu. Rev. Biochem., 74, pp.711-738.
- Friedmann, T., 1992. A brief history of gene therapy. Nature genetics, 2(2), pp.93-98.

