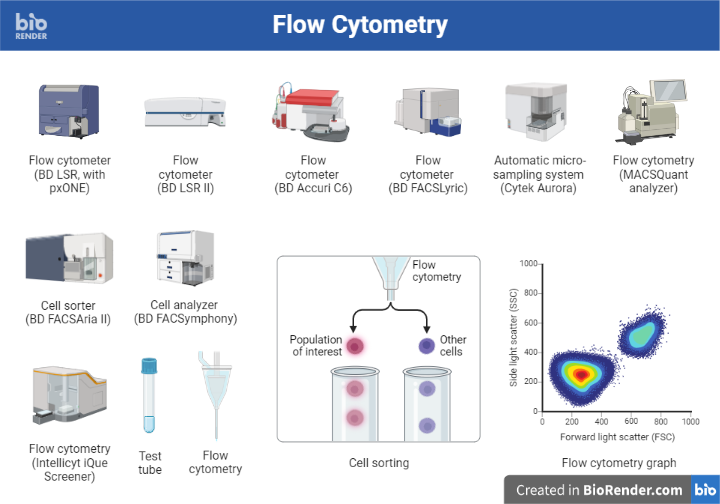
Introduction:
Flow cytometry is a technique used to measure and analyse the physical and chemical characteristics of cells or particles suspended in a fluid. It is commonly used in biological and medical research to analyse the properties of cells, including size, shape, and surface markers, as well as to sort cells into different populations based on these properties.
Fluorescence-activated cell sorting (FACS) is a technique used to isolate and purify specific populations of cells from a mixture based on their fluorescence characteristics. It is commonly used in cell biology and immunology research to study the function and behaviours of different cell types.
Fluorescence-activated cell sorting (FACS), also known as fluorescence-assisted cell sorting, is a kind of flow cytometry that targets and isolates cell groupings using fluorescent markers. This cell sorting approach is widely utilized in studies on haematopoiesis, cancer, and stem cell biology.
Principle:
The principle of flow cytometry is based on the measurement of light scattered or emitted by cells as they pass through a laser beam.
In a flow cytometry experiment, a sample of cells is suspended in a fluid and passed through a flow cytometer, which uses lasers to illuminate the cells and detect the light scattered or emitted by the cells. The flow cytometer is equipped with photodetectors that are positioned at different angles relative to the laser beam, allowing them to detect light scattered at different angles. The detected light is then analyzed by a computer to determine the size, shape, and complexity of the cells, as well as to identify specific surface markers or intracellular molecules.
However, in case with FACS it is based on the measurement of fluorescence intensity and wavelength emitted by cells that have been labeled with fluorescent probes.
Moreover, cells are first labeled with fluorescent probes that bind to specific proteins or other cellular components of interest. The labeled cells are then suspended in a fluid and passed through a flow cytometer, which uses lasers to excite the fluorescent probes and measure the resulting fluorescence. Based on the intensity and wavelength of the fluorescence, the flow cytometer can sort the cells into different populations.
The sorting of cells is achieved through the use of specialized optics and electronics that detect the fluorescence emitted by the cells and use this information to direct the cells into different channels or tubes. For example, cells with high levels of fluorescence may be directed into one tube, while cells with low levels of fluorescence may be directed into another tube.
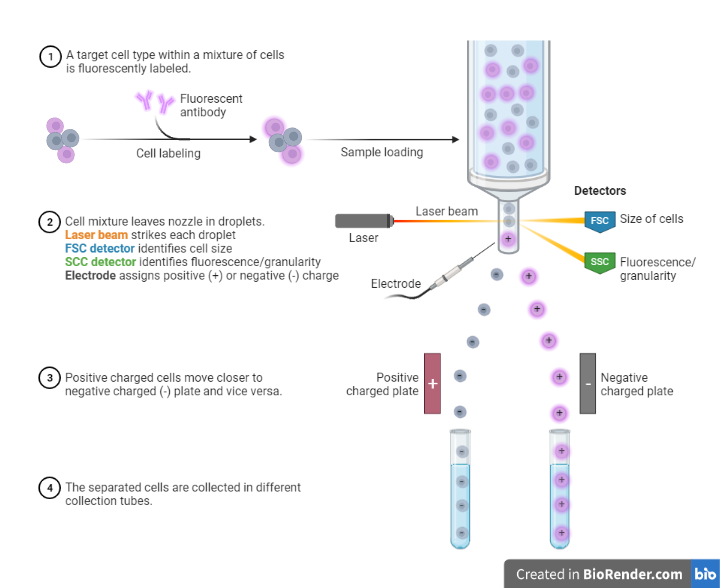
Fig: Principle of FACS
Parts:
Fluorescent probes
These are molecules that bind to specific proteins or other cellular components of interest and emit fluorescence when excited by lasers or other light sources. Different probes are available for different applications, and researchers can choose probes that are specific to the proteins or components they want to study.
Flow cytometer
This is the main instrument used in FACS. It consists of a flow cell, lasers or other light sources, detectors, and electronics that control the flow of cells through the flow cell and measure the fluorescence emitted by the cells.
A flow cytometer typically uses one or more lasers to illuminate the cells in the sample. Different lasers are used to excite different fluorescent labels, allowing the flow cytometer to analyzed multiple markers simultaneously.
Filter system
This is a set of filters that is used to separate the emitted or scattered light into different wavelengths or colours. The filters are used to distinguish the fluorescence emitted by different labels, allowing the flow cytometer to analyzed multiple markers simultaneously.
Sorting optics
These are specialized optics that detect the fluorescence emitted by the cells and use this information to direct the cells into different channels or tubes.
Sorting tubes
These are tubes or channels that collect the sorted cells.
Data analysis software
This software is used to analyse the data generated by the flow cytometer, including the fluorescence intensity and wavelength of the cells, and to sort the cells into different populations based on these parameters.
Cell culture equipment
This includes incubators, cell culture flasks and dishes, and other equipment used to culture cells in the laboratory.
Steps:
The steps involved are as follows:
Cell preparation
The cells to be sorted are first collected and labeled with fluorescent probes that bind to specific proteins or other cellular components of interest. The cells may be collected from tissue samples, cell cultures, or other sources.
The procedure for cell preparation typically includes the following steps:
Cell collection: The cells to be sorted are collected from tissue samples, cell cultures, or other sources. The cells may be collected using standard techniques, such as mechanical disruption of tissue or centrifugation of cell cultures.
Cell viability: The viability of the cells is assessed using a viability dye, such as propidium iodide or trypan blue. This helps to ensure that only healthy, viable cells are sorted.
Cell washing: The cells are washed to remove any contaminants or debris that may interfere with the sorting process.
Cell counting: The number of cells is determined using a hemocytometer or other cell counting method.
Cell suspension: The cells are suspended in a suitable medium, such as phosphate-buffered saline (PBS), at a concentration suitable for sorting.
Fluorescent labeling
In case with FACS, the cells are labeled with fluorescent probes by incubating them with the probes in the presence of a crosslinking agent. The probes bind to the proteins or components of interest, and the crosslinking agent stabilizes the binding.
There are many different types of fluorescent probes available, including dyes, antibodies, and small molecules. The choice of probe depends on the protein or component being studied and the fluorescence characteristics of the probe.
Some commonly used fluorescent probes include:
Fluorescent dyes: These are small molecules that bind to specific proteins or components and emit fluorescence when excited by lasers. Examples include fluorescein, rhodamine, and phycoerythrin.
Fluorescent antibodies: These are antibodies that have been labeled with fluorescent probes. They bind to specific proteins and can be used to label cells or tissues for FACS analysis.
Fluorescent proteins: These are proteins that naturally emit fluorescence when excited by lasers. Examples include green fluorescent protein (GFP) and red fluorescent protein (RFP).
Loading the sample
The sample is loaded into a flow cytometer, which is a specialized instrument that uses lasers to analyzed the cells as they pass through a stream of fluid.
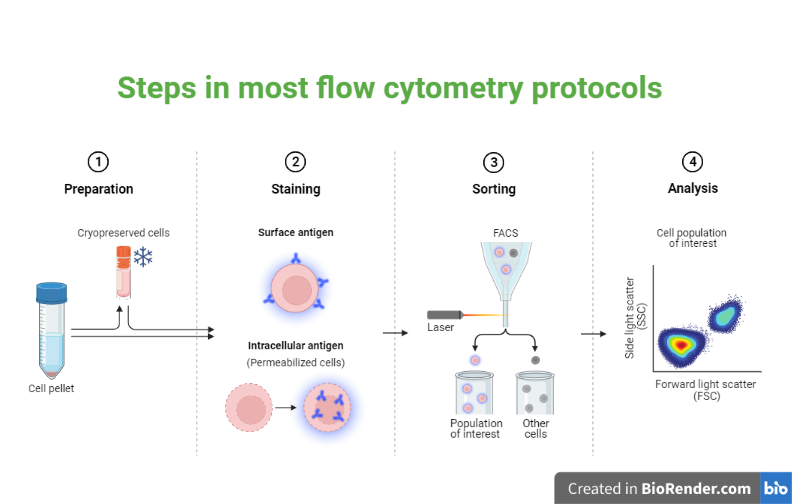
Fig: Steps in most flow cytometry protocols
Data acquisition
The labeled cells are then suspended in a fluid and passed through a flow cytometer, which uses lasers to excite the fluorescent probes and measure the resulting fluorescence. The flow cytometer generates data on the intensity and wavelength of the fluorescence emitted by the cells. The data is plotted on a scatterplot, with one axis representing fluorescence intensity and the other axis representing wavelength.
Data analysis
The data generated by the flow cytometer is analyzed using specialized software, which sorts the cells into different populations based on the intensity and wavelength of the fluorescence. The sorted cells are analyzed to determine their characteristics and functions, and to identify trends and patterns within the data. This may involve additional experimentation or analysis, such as gene expression analysis or functional assays.
Cell sorting
The sorted cells are collected into tubes or other containers for further analysis or experimentation. The sorting system uses lasers and mechanical devices to direct cells into different tubes or wells based on the data generated.
Types:
Static sorters
These systems sort cells by directing them into tubes or other containers using specialized optics. The cells are suspended in a fluid and passed through a flow cell, where they are excited by lasers or other light sources and their fluorescence is measured. The sorted cells are collected into tubes or other containers for further analysis or experimentation.
Continuous flow sorters
These systems sort cells by directing them into tubes or other containers using specialized optics and a stream of fluid. The cells are suspended in the fluid and passed through a flow cell, where they are excited by lasers or other light sources and their fluorescence is measured. The sorted cells are collected into tubes or other containers for further analysis or experimentation.
Cell sorter/analyser hybrids
These systems combine the features of static and continuous flow sorters, allowing researchers to both analyse and sort cells.
Multicolour sorters
These systems use multiple lasers and fluorescent probes to sort cells based on multiple fluorescence parameters simultaneously.
Single parameter sorting
This type of FACS uses a single fluorescence parameter, such as fluorescence intensity or wavelength, to sort cells. It is useful for purifying cells that express a single protein or component of interest.
Two-parameter sorting
This type of FACS uses two fluorescence parameters, such as fluorescence intensity and wavelength, to sort cells. It allows researchers to purify cells that express a combination of proteins or components.
Multiparameter sorting
This type of FACS uses three or more fluorescence parameters to sort cells. It allows researchers to purify cells that express a complex combination of proteins or components, and is particularly useful for studying the function and behaviours of cells in more detail.
Applications:
Cancer research: It is used to purify and isolate specific populations of cancer cells for further study. It can also be used to identify and characterize cancer stem cells, which are thought to be responsible for the development and progression of cancer.
Stem cell research: It is used to purify and isolate specific populations of stem cells for further study. It can also be used to identify and characterize different types of stem cells, such as embryonic stem cells and adult stem cells.
Analysis of immune cell populations: It is widely used in the field of immunology to study immune cell populations and to identify rare cells, such as cancer cells or cells infected with a virus.
Blood cell analysis: It is used to analyzed blood cells, including red blood cells, white blood cells, and platelets. It can be used to diagnose blood disorders, such as anemia or leukemia, and to monitor the effectiveness of treatments.
Immune system studies: It is used to purify and isolate specific populations of immune cells, such as T cells and B cells, for further study. It is also used to study the function and behaviours of these cells in the context of immune system disorders, such as autoimmune diseases and allergies.
Diagnosis and monitoring of diseases: It is used in clinical settings to diagnose and monitor diseases, such as leukemia and lymphoma. It can also be used to monitor the effectiveness of treatments for these diseases.
Tissue engineering and regenerative medicine: It is used to purify and isolate specific populations of cells for use in tissue engineering and regenerative medicine applications.
Drug development: FACS is used to study the effects of drugs on specific cell populations, helping to identify potential new drugs and to optimize their effectiveness.
Limitations:
Expense: It requires specialized equipment and personnel, which can be expensive.
Complexity: It requires a high level of technical expertise, which may not be available in all laboratories. It also requires the use of specialized software and data analysis techniques, which may be complex and time-consuming.
Sample preparation: It requires that cells be labeled with fluorescent probes, which may be difficult or time-consuming to prepare. In addition, the labeling process may affect the function or behaviours of the cells being studied.
Multiple fluorescence parameters: It can sort cells based on multiple fluorescence parameters, but this can be challenging and may require the use of specialized software and data analysis techniques.
Contamination: It requires a cleanroom environment to minimize contamination, which can be challenging to maintain.
References:
- Fu, A.Y., Spence, C., Scherer, A., Arnold, F.H. and Quake, S.R., 1999. A microfabricated fluorescence-activated cell sorter. Nature biotechnology, 17(11), pp.1109-1111.
- McKinnon, K.M., 2018. Flow cytometry: an overview. Current protocols in immunology, 120(1), pp.5-1.
- Sharrow, S.O., 2002. Overview of flow cytometry. Current Protocols in Immunology, 50(1), pp.5-1.
- Robinson, J.P., 2018. Overview of flow cytometry and microbiology. Current protocols in cytometry, 84(1), p.e37.
- Büscher, M., 2019. Flow cytometry instrumentation–an overview. Current Protocols in Cytometry, 87(1), p.e52.