Factors Affecting Enzyme Activity:
Substrate concentration
- The increase in velocity is proportional to the substrate concentration.
- When substrate concentration is higher, reaction is faster but it reaches a saturation point when all the enzyme molecules are occupied.
- If the concentration of the enzyme is altered then Vmax will change too.
- Within the constrained range of substrate levels, an increase in substrate concentration progressively speeds up the enzyme process.
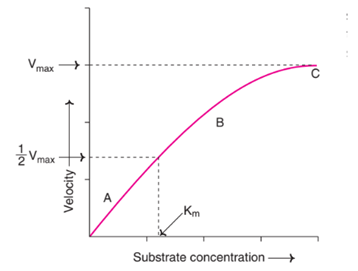
Fig: Effect of substrate concentration on enzyme velocity
Enzyme concentration
- The velocity of the enzymatic reaction is directly proportional to the enzyme concentration in the beginning.
- When the substrate concentration is in large excess exceeding that of Vmax, more enzyme molecules are provided to enable the conversion of progressively larger number of substrate molecules.
- The enzyme might be evaluated in the lab using a known volume of blood and maintaining optimal levels of all other parameters (substrate, pH, temperature, etc.).
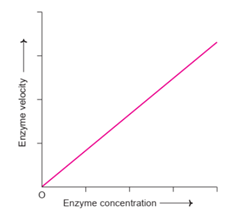
Fig: Effect of enzyme concentration on enzyme velocity
Effect of pH
- The enzyme activity is significantly impacted by an increase in the hydrogen ion concentration (pH), and a bell-shaped curve is typically produced.
- Every enzyme has a pH range where its greatest velocity occurs.
- The majority of higher organisms’ enzymes function best at neutral pH values (6–8).
A few exceptions include alkaline phosphatase (10–11), acid phosphatase (4-5), and pepsin (1-2).
- Fungi and plant enzymes are most active in acidic pH ranges (4-6).
- By changing the ionic charges on the amino acids, hydrogen ions affect the activity of the enzyme (particularly at the active site), substrate, ES complex etc.
- Extreme pH levels will produce denaturation.
- The structure of the enzyme is changed.
- The active site is distorted and the substrate molecules will no longer fit in it.
Effect of temperature
• An enzyme reaction’s velocity rises with temperature until it reaches its maximum and then falls.
• Typically, a bell-shaped curve is seen.
• Most enzymes function best at temperatures between 35°C and 40°C.
• Since their enzymes denature at 30°C, many cold-water fish will perish.
• Enzymes found in certain bacteria may tolerate temperatures as high as 100°C.
The temperature coefficient (Q₁₀) refers to how much the rate of an enzymatic reaction speeds up when the temperature is increased by 10 °C.
For the majority of enzymes, the Q₁₀ value is observed within the temperature range of 0 °C to 40 °C.
Denaturation, which results in a disruption of the protein’s native (tertiary) structure and active site, occurs at temperatures higher than 50°C.
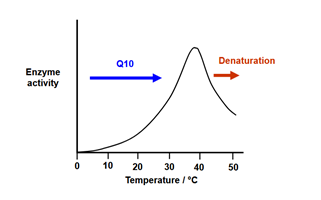
Fig: Effect of temperature on enzyme activity
Effect of activators
• Some of the enzymes require certain inorganic metallic cations like Mg2+, Mn2+, zn2+, ca2+, co2+,
cu2+, Na+, K+ etc” for their optimum activity.
• Rarely, anions are also needed for enzyme activity e.g. chloride ion (C1-) for amylase.
• Metals function as activators of enzyme velocity through various mechanisms- combining with the
substrate, formation of ES-metal complex, direct participation in the reaction and bringing a
conformational change in the enzyme.
The two types of enzymes that need metals to function are separated.
Metal-activated enzymes such as ATPase (requiring Mg²⁺ or Ca²⁺) and Enolase (requiring Mg²⁺) use metal ions loosely bound to their structure. These ions can be easily replaced or exchanged with others.
In contrast, metalloenzymes bind their metal ions tightly, making them far less interchangeable.
Examples of such enzymes include alkaline phosphatase, carboxypeptidase, aldolase, carbonic anhydrase, and alcohol dehydrogenase, all of which incorporate zinc as a cofactor.
• Phenol oxidase (copper);
• Pyruvate oxidase (manganese);
• Xanthine oxidase (molybdenum);
• Cytochrome oxidase (iron and copper)
Effect of time
- Under ideal and optimal conditions (like pH, temperature, etc) the time required for an enzyme reaction is less.
- Variations in the time of the reaction are generally related to the alterations in pH, and temperature.
Effect of light and radiation
- Some enzymes are sensitive to light.
- Some enzymes require the presence of light for its catalytic activity.
Example: The enzyme photolyase, which plays a key role in photoreactivation DNA repair, becomes active only in the presence of light.
When enzymes are exposed to high-energy radiations such as ultraviolet rays, beta rays, gamma rays, or X-rays, they may lose their activity because these radiations can damage their structure.
Importance of enzyme:
- The presence and maintenance of a complete and balanced set of enzymes is essential for the break down off nutrients to supply energy and chemical building blocks.
- Deficiency in the quantity or catalytic activity of key enzymes can result from genetic defects, nutritional deficits or toxins.
- Defective enzymes can result from genetic mutations or infections by viral or bacterial pathogens.
- Measurement of enzyme in the blood is used for the diagnosis.
- Many drugs are designed to target enzymes of the cells.
- Enzymes are also used as drugs.
Allosteric enzymes:
Allosteric enzymes are a special class of enzymes that alter their three-dimensional structure when an effector molecule (also called an allosteric modulator) binds to them. This structural adjustment changes how strongly the enzyme interacts with a ligand at a separate binding site.
- These enzymes contain an additional region termed the allosteric site, distinct from the active site.
- Regulation takes place when certain molecules attach to this allosteric site, thereby modifying the enzyme’s activity without directly competing with the substrate.
- The molecule responsible for this modulation is referred to as the allosteric effector.
If a negative effector binds to the allosteric site, the enzyme’s activity is reduced or inhibited.
Binding of an effector at one site triggers a structural shift in that subunit, which in turn induces similar conformational adjustments in the other subunits of the enzyme.
Much of the effector’s binding energy is therefore invested in reshaping the entire protein complex, rather than just one part of it.
