Introduction:
The electron transport chain (ETC) is a series of protein complexes and electron carriers that are located in the inner membrane of mitochondria. It is an important process that occurs in cells and is involved in the production of ATP (adenosine triphosphate), the main energy currency of the cell.
The component that generates energy for metabolic functions in our cells is called ATP (Adenosine Triphosphate) which is only possible when a molecular unit of adenosine gets connected to three phosphate groups.
A phosphoryl group (PO3-), which is added chemically to an organic molecule, is phosphorylation. In contrast, dephosphorylation is the process by which a phosphoryl group is removed. Enzymes are responsible for both phosphorylation and dephosphorylation (e.g., kinases, phosphotransferases).
Phosphorylation is significant in the fields of biochemistry and molecular biology because of its crucial reaction in the functioning of proteins and enzymes, sugar metabolism, and the storage and release of energy.
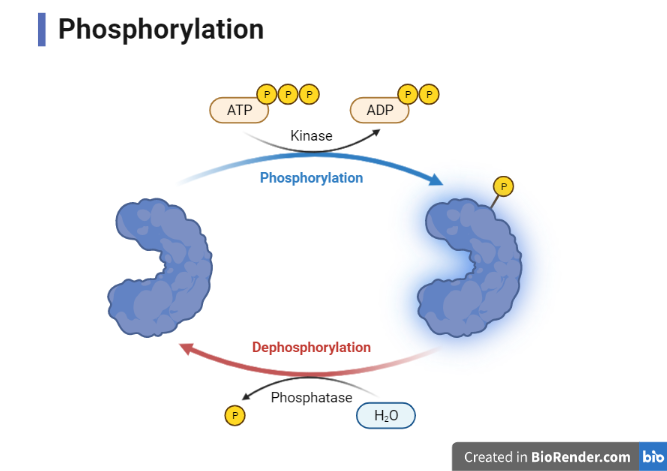
Fig: Phosphorylation
Purposes of Phosphorylation:
Phosphorylation plays a critical regulatory role in cells. Its functions include:
- Crucial to glycolysis
- For interacting with proteins
- Protein breakdown process
- Control of enzyme inhibition
- It controls chemical reactions that need energy in order to maintain homeostasis.
Types:
Protein Phosphorylation
The most prevalent approach to controlling protein activity and conveying signals throughout the cell is phosphorylation.
Although phosphorylation has been seen in bacterial proteins, it is much more common in eukaryotic cells.
One third of the human proteins are believed to be substrates for phosphorylation at a certain stage.
The regulation of numerous cellular processes, such as the cell cycle, proliferation, apoptosis, and signal transduction pathways, is significantly assisted by the existence of phosphorylation.
Mechanism of phosphorylation
Although phosphorylation is a common post-translational modification (PTM) for controlling protein activity, it only happens at the side chains of the three amino acids serine, threonine, and tyrosine in eukaryotic cells.
The universal phosphoryl donor adenosine triphosphate (ATP) is composed of these amino acids, which have a nucleophilic (-OH) group that attacks the terminal phosphate group and causes the transfer of the phosphate group to the amino acid side chain in the aid of magnesium (Mg2+).
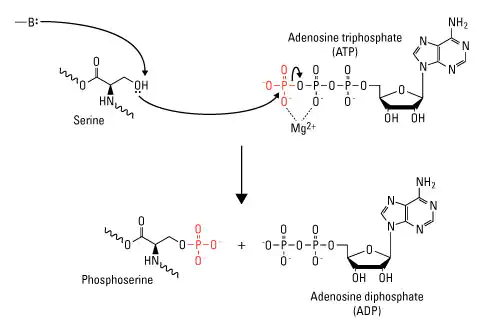
Fig: Protein phosphorylation
Substrate level phosphorylation (SLP)
Substrate level phosphorylation is a metabolic mechanism of ATP production involving the transfer of a phosphate (Pi) from a donor molecule to ADP to form ATP.
Through this process most of our ATP care created inside mitochondria but some ATP can also be made in the cytoplasm. It is a faster but less effective source of ATP than oxidative phosphorylation and can take place in both aerobic and anaerobic conditions.
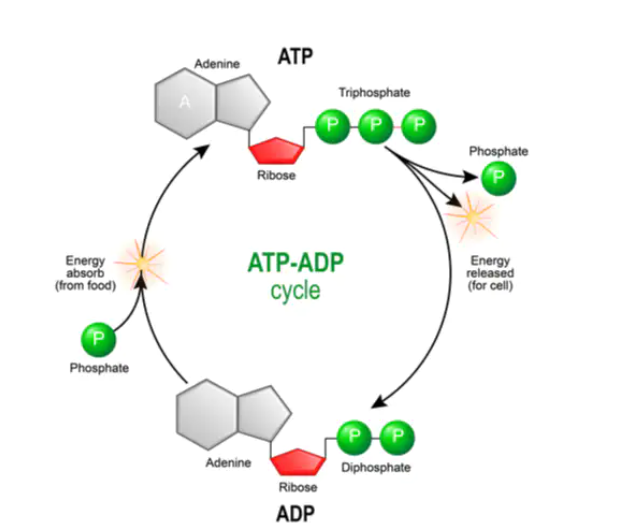
Fig: ATP-ADP Cycle in substrate level phosphorylation
Through phosphoglycerate kinase, 1,3-bisphosphoglycerate is dephosphorylated, resulting in the phosphorylation of the substrate level to produce 3-phosphoglycerate and ATP.
By dephosphorylating phosphoenolpyruvate, which is carried out by pyruvate kinase and results in the production of pyruvate and ATP.
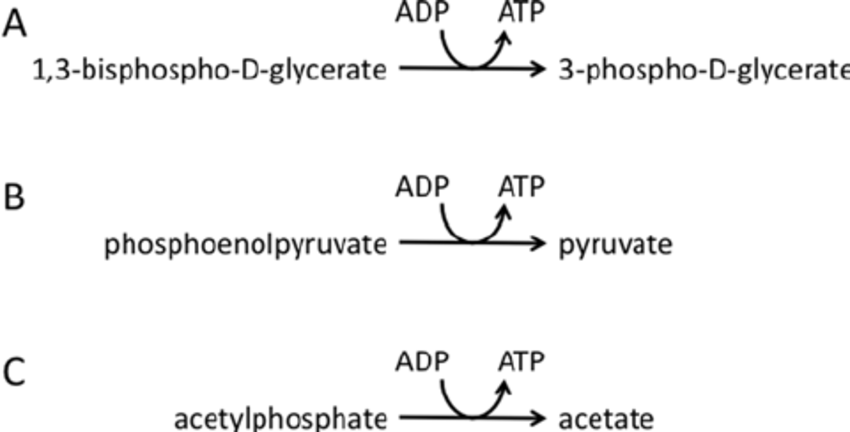
Fig: Examples of substrate level phosphorylation
Oxidative phosphorylation
The component of aerobic respiration known as the electron transport chain consists of a series of four protein complexes that couple redox processes and create an electrochemical gradient that results in the production of ATP in a whole process known as oxidative phosphorylation.
Oxidative phosphorylation has two parts:
Electron Transport Chain (ETC)
The ETC is composed of a group of proteins that are anchored to the inner mitochondrial membrane and organic molecules that allow electrons to pass through and release energy through a series of redox processes. The protein ATP-synthase complex uses the energy liberated to establish a proton gradient, which is then utilised in chemiosmosis to produce a significant amount of ATP.
It is the final stage of aerobic respiration and the only process that utilises ambient oxygen in the metabolism of glucose.
In eukaryotes, the complexes are located within the cristae, the inner mitochondrial membrane. The matrix, which is surrounded by the inner mitochondrial membrane, is where vital enzymes like pyruvate dehydrogenase and pyruvate carboxylase are located.
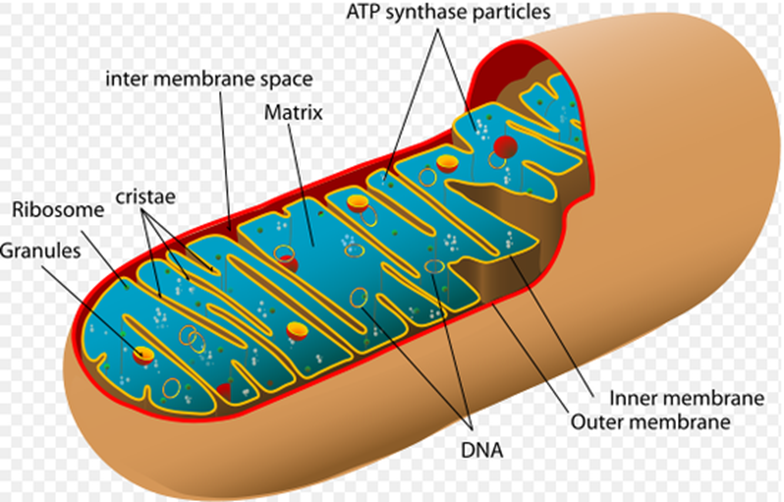
Fig: Structure of mitochondria
The electrons in the electron transport chain (ETC) pass through a chain of proteins that increases its reduction potential and results in an energy release.
It is a sequence of redox processes in which electrons are quickly transferred from one part to the other, where they reduce molecule of oxygen to produce water.
The electron transport chain consists of the aggregation of the four protein complexes, designated I through IV, and their associated mobile, accessory electron carriers.
Both the plasma membrane of prokaryotes and the inner mitochondrial membrane of eukaryotes include several copies of the electron transport chain.
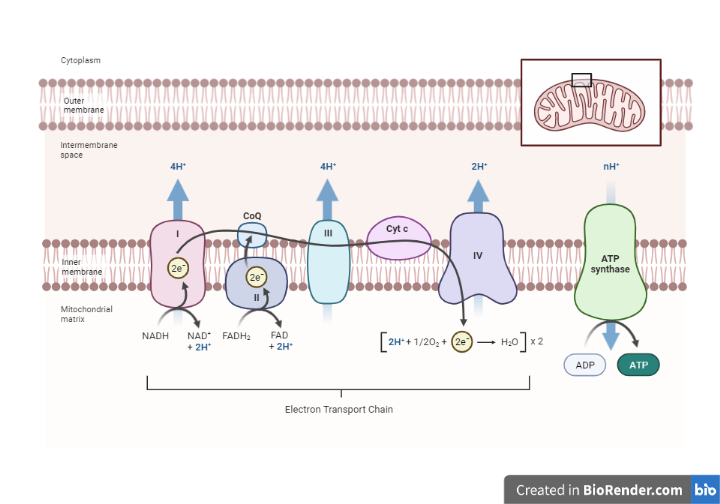
Fig: Overview oxidative phosphorylation
Nicotinamide nucleotides, flavo proteins, iron-Sulfur proteins, coenzyme Q and cytochromes are the carrier protein that participate in the chain.
These carriers, which are sequentially arranged, are in responsible for transporting e- from one substrate to another, where it eventually combines with proton and oxygen to produce H2O.
Complex I: NADH-Coenzyme Q Oxidoreductase
It is constituted of a protein containing iron-sulphur (Fe-S) and flavin mononucleotide (FMN).
A number of prosthetic groups or co-factors are present in the electron transport chain, including FMN, which is generated from vitamin B2, often known as riboflavin. NADH dehydrogenase, an extensive protein enzyme, is the component of complex I.
The hydrogen ion gradient between the two compartments divided by the inner mitochondrial membrane is produced and controlled by the ability of this complex to pump four hydrogen ions from the matrix across the membrane and into the intermembrane region.
Complex II: Succinate-Coenzyme Q Oxidoreductase
FADH2 is promptly absorbed by complex II. Ubiquinone (Q) is the substance that joins the first and second complexes to the third. The Q molecule may freely pass through the hydrophobic centre of the membrane because it is lipid soluble. Q receives the FADH2 electrons from complex II, which also contains succinate dehydrogenase, and the NADH electrons from complex I. The number of protons that are finally pumped across the inner mitochondrial membrane determines final concentration of ATP molecules that are synthesized.
Complex III: Cytochrome bc1 Oxidoreductase
The third complex, also known as cytochrome oxidoreductase, is comprised of cytochrome b, an additional Fe-S protein, and cytochrome c proteins. Heme forms a prosthetic group on cytochrome proteins. Complex III transports its electrons to cytochrome c for transfer to the fourth complex of proteins and enzymes while pumping protons through the membrane. The acceptor of electrons from Q is cytochrome c.
Complex IV: Cytochrome c Oxidase
The cytochrome proteins c, a, and a3 make up the fourth complex. The oxygen is held between the iron and copper ions by the cytochromes in a very stable way until the oxygen is entirely reduced. The consequent water is produced when the reduced oxygen picks up two hydrogen ions from the medium (H2O). The ion gradient employed in the chemiosmosis process is influenced by the removal of hydrogen ions from the environment.
ATP synthase complex
The ATP synthase complex is a vital enzyme complex found in eukaryotic cells’ inner mitochondrial membranes and bacteria’s plasma membranes. It is made up of the F1 and Fo complexes and is essential for cellular energy production. The F1 complex contains catalytic subunits responsible for ATP production and is found within the mitochondrial matrix or cytoplasm. The Fo complex, on the other hand, spans the membrane and functions as a proton channel, allowing protons to flow back into the matrix or cytoplasm. The ATP synthase complex uses the electrochemical gradient of protons generated during electron transport chain reactions to synthesis ATP from ADP and Pi.
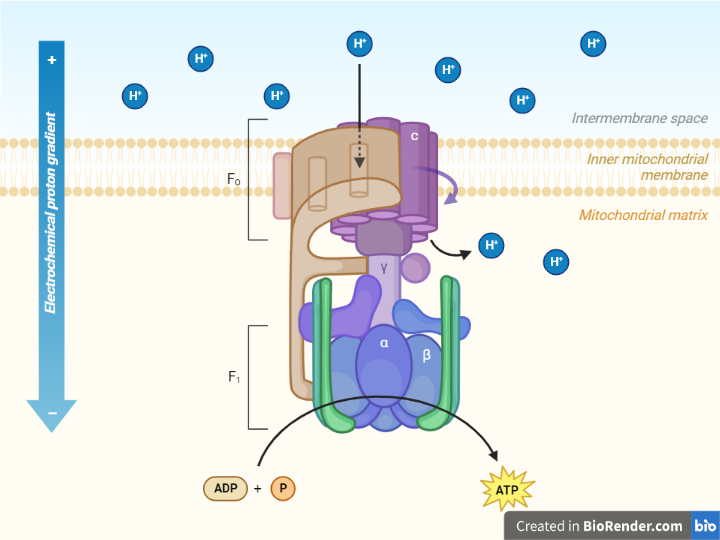
Fig: Structure of ATP Synthase complex
The energy from the proton gradient is converted into ATP as the ATP synthase rotates, catalyzing the addition of a phosphate to ADP.
The F1 sector, a soluble part located in the mitochondrial matrix, and the Fo sector, which is bound to the inner mitochondrial membrane, are the two distinct protein entities that constitute ATP synthase.
Chemiosmosis
Proton pumps are found in complexes I, III, and IV of the electron transport chain. As the electrons migrate energetically downward towards the chain, the H+ ion will eventually pump to the intermembrane space. Thus, the inner mitochondrial membrane develops an electrochemical gradient as a result of this pumping. The gradient may also be referred to as the proton-motive force (PMF).
Protons can’t immediately flow through the phospholipid bilayer of the membrane because its core is too hydrophobic, like many other ions. Instead, H+ ions can only travel along their concentration gradient through the channel proteins that create hydrophilic tunnels across the membrane which is known as ATP synthase complex. ATP synthase is similar in concept to a turbine in a hydroelectric power plant. It is turned by the flow of H+ ions traveling down their electrochemical gradient as opposed to being turned by water.
Clinical Significances:
- The electron transport chain (ETC), which is made up of protein complexes, enables the synthesis of ATP which always remain as the fundamental unit of energy in all living things.
- Neurodegenerative illnesses like Parkinson’s disease (PD) are connected to defects in ETC function, which have wide-ranging effects. In both sporadic and familial cases of PD, defects in NADH: ubiquinone oxidoreductase, the first complex in the ETC, have been discovered.
- Succinate dehydrogenase gene mutations can result in decreased electron flow and increased oxygen toxicity. These effects can appear clinically in a variety of ways in people, including the encephalomyopathy, tumours, and optic atrophy that characterize Leigh syndrome.
References:
- Kresge, Nicole; Simoni, Robert D.; Hill, Robert L. (2011-01-21). “The Process of Reversible Phosphorylation: the Work of Edmond H. Fischer”. Journal of Biological Chemistry. 286 (3).
- Jain JL, Jain S, and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- Sharma, Saumya; Guthrie, Patrick H.; Chan, Suzanne S.; Haq, Syed; Taegtmeyer, Heinrich (2007-10-01). “Glucose Phosphorylation Is Required for Insulin-Dependent mTOR Signalling in the Heart”. Cardiovascular Research. 76 (1): 71–80.
- Lencina AM, Franza T, Sullivan MJ, Ulett GC, Ipe DS, Gaudu P, Gennis RB, Schurig-Briccio LA. Type 2 NADH Dehydrogenase Is the Only Point of Entry for Electrons into the Streptococcus agalactiae Respiratory Chain and Is a Potential Drug Target. mBio. 2018 Jul 03;9(4)
- David, L., Nelson, D.L., Cox, M.M., Stiedemann, L., McGlynn Jr, M.E. and Fay, M.R., 2000. Lehninger principles of biochemistry.
- Boyer R, Concepts in Biochemistry, 3rd edition. New Jersey: John Wiley & Sons; 2006.
