Difference between Class I and Class II MHC molecule:
| Properties | Class I MHC molecule | Class II MHC molecule |
| Polypeptide chain | It has α chain and β2 microglobulin. | It consists of α chain and β chains. |
| Binding site of peptides | Cleft of of α1 and α2 chains. | Cleft of α1 and β1 chain |
| Coded by | Class I MHC gene | Class II MHC gene |
| Polymorphic residues found in | α1 and α2 domains | α1 and β1 domains |
| Capacity of loading peptides that can be loaded in cleft | Peptides of 8-10 amino acid residues | Peptides of 10-30 amino acid residues |
| Binding site of T cell coreceptor | CD8 binds to α3 region | CD4 binds to β2 region. |
| Antigen loaded on MHC presented to | CD8+ T cells | CD4+ T cells |
| Nomenclature in human | HLA-A, HLA-B, HLA-C | HLA-DR, HLA-DQ, HLA-DP |
The endocytic pathway for antigen processing and presentation:
The extracellular protein antigens that are absorbed by Antigen Processing Cells (APC) in the host endocytic vesicles undergo proteolytic digestion as part of the endocytic cascade of antigen processing. T-helper cells (CD4+ T cells) see the protein antigens after they have been broken down into smaller peptides and loaded onto Class II Major Histocompatibility Class (MHC) molecules.
To show up on the APC, extracellular protein antigens bind to the surface receptors of several APCs, including dendritic cells and macrophages.
By identifying the components that microorganisms share, different APC can bind protein antigens in a variety of ways, with differing specificities and efficiencies.
Endosomes are membrane-bound vesicles that are responsible for the engulfment and localization of protein antigens. The three ever more acidic compartments in the endosomal pathway include lysosomes (pH 4.5–5.0), late endosomes, also called endolysosomes (pH 5.0–6.0), and early endosomes (pH 6.0–6.5).
The engulfed antigen moves from early to late endosomes and finally to lysosomes where hydrolytic enzymes and a lower pH are present in each compartment. The steps included in this pathway are:
Class II MHC Molecule Biosynthesis and Endosome Transport:
Class II MHC molecules are synthesized in the Rough Endoplasmic Reticulum (RER) and transported to endosomes along with an associated protein, the invariant chain (Ii), which occupies the peptide-binding clefts of the newly synthesized class II molecules. The α and β are formed and joined together within the endoplasmic reticulum (ER). These newly formed heterodimers are initially unstable and require assistance from ER-resident chaperones like calnexin for proper folding and assembly. A critical player in this process is the invariant chain (Ii), which not only stabilizes the αβ complex but also ensures its correct folding. The class II-invariant chain complexes are then packaged into exocytic vesicles and sent toward the cell surface. En route, these vesicles merge with endocytic compartments rich in peptides derived from degraded extracellular proteins. This fusion enables the class II molecules to finally acquire their antigenic peptides before being displayed on the cell membrane.
Association of Processed Peptides with Class II MHC Molecules in Vesicles:
After cathepsins (protease) work on the Ii within the endosomal vesicles, the only residue that remains is Class II–associated Invariant Chain Peptide (CLIP), a 24-amino acid remnant that fills the peptide-binding cleft in the same way that other peptides attach to class II MHC molecules. In order for other extracellular proteins to bind CLIP, it is eliminated.
This is facilitated by the reaction between HLA-DM and Class II-CLIP complex.
Peptides produced by the proteolysis of absorbed protein antigens can attach to class II MHC molecules after CLIP is eliminated. After being “trimmed” by proteolytic enzymes to the appropriate size for T cell recognition, large peptides may bind because of the open ends of the class II MHC peptide binding cleft. Ten to thirty amino acids make up the loaded peptides that are produced.
Cell Surface Expression of Peptide–Class II MHC Complexes:
The process by which vesicular-tubular extensions from the lysosome fuse with the plasma membrane to transfer the loaded class II MHC complexes to the cell surface for presentation to CD4 cells is known as transport of class II MHC–peptide complexes. The CD4 coreceptor is used by peptide antigen-specific CD4+ T cells to recognize the peptide–class II complexes produced on the APC.
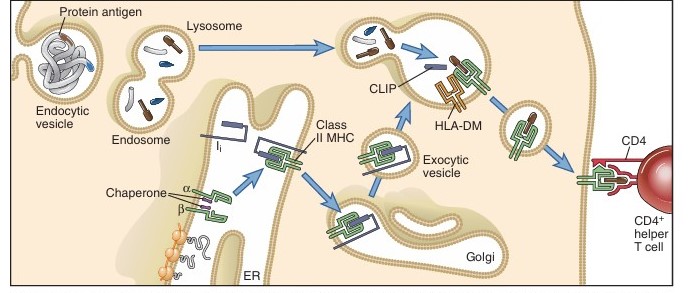
Fig: The class II MHC pathway of antigen presentation
Source: Abbas, A. K., Lichtman, A. H., & Pillai, S. (2012). Cellular and molecular immunology. 7th ed. Elsevier/Saunders.
References
- Abbas, A. K., Lichtman, A. H., & Pillai, S. (2012). Cellular and molecular immunology. 7th ed. Elsevier/Saunders.
