Introduction to Cell Cycle Regulation:
Cell division is a very crucial phenomenon for normal growth, development and maintenance. But in a body of eukaryotes organisms such as humans, the rate of timing of cell division varies on the type of cells.
For e.g., skin cells divide frequently throughout life time. However, specialized cells such as nerve cells don’t divide at all. The reason behind these differences of frequency of cell division is regulation of cell cycle at molecular level.
Cell cycle is regulated by both external and internal signals. In other words, the cell cycle control system is made up of external signals received by a cell and cells internal environment.
Signals of cell cycle control:
External Signals in Cell Cycle Control
We understand that the cells of the human body communicate with each other. Cell communication is a part of the regulation and coordination system. Cell-cell communicate, signal each other and thus Convey what to do. The signals (signaling molecules) that regulate and coordinate cell proliferation throughout the body are known as growth factors. The growth factors are the extracellular signals that inhibit or stimulate cell proliferation. It is also known as mitogens. There are several types of growth factors such as Epidermal Growth Factor (EGF), Platelet-Derived Growth Factor (PDGF), Fibroblast Growth Factor (FGF), Transforming Growth Factor-β (TGF-β). The several types of growth factors bind to various types of receptors for cell signaling.
EGF secreted by endocrine cells stimulate epidermal cells such as skin cells and regulate whether to proliferate or not. Therefore, growth factors bind to the receptor in the cell and the binding stimulates signaling transduction cascade inside the cell.
Signal transduction cascade is a series of biochemical reactions that convey signals from outside of a cell to its nucleus. Inside the nucleus, this signal activates a gene that encodes transcription factors (TFs). TFS are proteins that control the rate of transcription of genetic information. TFs either activate or repress the expression of another set of specific genes. These genes are those whose product will either promote or inhibit cell division. TFs regulate cell cycle genes. Most important cell cycle genes are activated in response to growth factors that transcribe the cyclins and cyclin-dependent kinases (CDK). CDK is a cytosolic protein, a machinery protein that drives cell cycle.
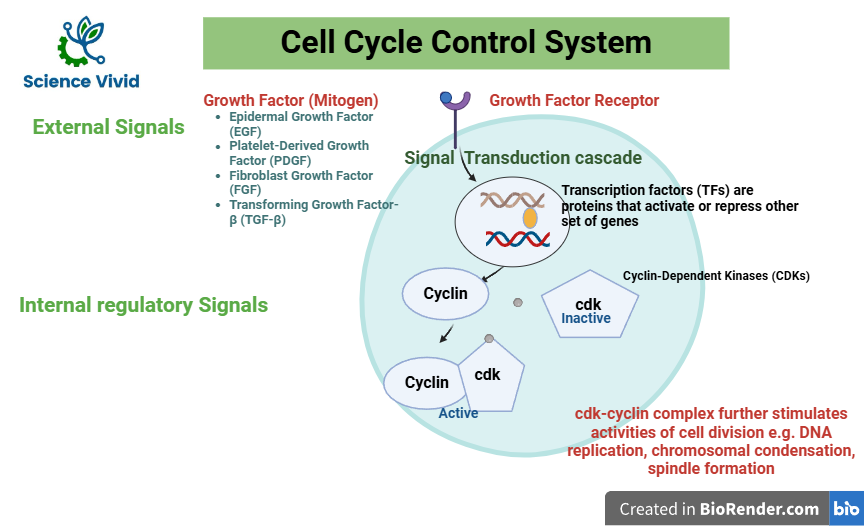
Fig: Cell cycle and Checkpoints: Introduction, External and Internal signals of cell cycle control
Internal regulatory signals of cell cycle control system
To ensure proper passage through the various phases (G1, S, G2, and M), the cell cycle is strictly controlled by internal checkpoints and chemical signals. The cyclins, cyclin-dependent kinases (CDKs), CDK inhibitors (CKIs), and checkpoint proteins are examples of these internal signals that keep an eye on cellular parameters like cell growth, chromosome alignment, and DNA integrity.
Protein kinases are the enzymes that activate or inactivate other proteins. They do these by phosphorylation. They are present in constant concentration in a growing cell. But, most of the time they are present in the active form. However, they are activated only when they are associated with cyclins. Cyclins is a protein and it is named so because its concentration fluctuates cyclically in the cell. As the concentration of cyclin varies, the activity of cdk also varies.
Thus, the cyclin and cdk are the proteins that are responsible for guiding cells from one phase of the cell cycle to the next.
Cyclins: Types and Regulation in Cell Cycle Control
Cyclins are proteins which binds to enzymes called cyclin dependent kinases (CKD). Many types of cyclins exist which bind to different types of CKDs.Cyclins are the protein that undergo a cyclical pattern of synthesis and degradation during the cell cycle. Hence, these are known as cyclins. So, in certain stages of cell cycle these are produced and in certain stages of cell cycle they are degraded. So, their appearance and activities are restricted in a time bound fashion. And this is how they are named as cyclins. Because their timely rise and falls in a coordinated manner with a progression of cell cycle.
Cyclin-dependent kinases (CDKs):
CKDs are enzymes which activate the proteins required for progression throughput the cell cycle and its checkpoints.These are the kinase molecules which are capable of phosphorylation. But they are highly dependent on their partner i.e., cyclin. It is like a couple; without cyclin it can’t work. So, they are highly dependent on each other and act together as a unit. They possess important functions which are regulated by CDks. At a structural level, serine/threonine kinases, i.es that phosphorylates serine and threonine residues in the target protein. Their activity is dependent on binding to the cyclins and without the cyclin they are functionless and can’t do anything. CDKs regulate the cell cycle by phosphorylating target protein involved in various cell cycle events and the activity of cyclins-CDKs complex is tightly controlled and regulated by action of several kinases and phosphoprotein.
Types of cyclins:
G1 CYCLIN (CYCLIN D-CDK4/6)
This complex will get activated where there are ample amounts of growth factors signaling. Cyclin D is directly produced in response to mitogenic signaling or growth factor signaling. So, it tells us about the presence of growth factors in the environment or not. If it is present in the environment then cyclin D will be produced, then the complex would be activated.
For e.g., we know that mitogen-activated protein kinase (MAPK) pathway is triggered by growth factor binding. So, obviously one of the key downstream genes that gets activated in the Ras/Raf/MAPK pathway is cyclin D. It is one of the most abundant targeted genes in the pathway.
Now, it is very important to note that whether to divide or not divide is a very critical decision that each cell has to make.
Because it is an energy dependent event, such as DNA replication in a S-phase, chromosome segregation in a M-phase. All of the events required a sufficient amount of energy. If the nutrient is present, it is an appropriate time to divide. So, there is a point in a cell cycle called a restriction point (R) where Cyclin D-CDK4/6 takes a critical decision. They took all the information from the growth factor signaling, nutrient availability and stress factor. Based on these factors, it decides whether a cell should cross the restriction point or shouldn’t cross. If a restriction point is crossed, it has to divide and there is no point to back out. Therefore, the activities of cyclin D-CDK4/6 are very important and crucial.
pRB in the cell cycle regulation and retinoblastoma
The Rb protein is a tumor suppressor protein that regulates the cell cycle progression or growth. It plays a pivotal role in the negative control of the cell cycle and in tumor progression. It is produced by the RB1 gene. pRB protein acts in a restriction protein at the restriction point (R point). pRB is a critical decision maker and can actually inhibit a compound known as E2F. E2F is really important for cell progression to S-phase. Because, it can selectively bind to DNA and help to produce the S-phase cyclin. But it can only do so when pRB is separated byE2F. So, separation of E2F is very crucial.
The cyclin D-CDK4/6 is responsible for the separation. If the environment is favorable, growth factor is presence, the cycle has enough nutrients then, cyclin D-CDK4/6 would phosphorylate pRB. Thus, phosphorylating pRB would allow the E2F complex.
P53 is a tumor suppressor protein that play a key role in
- Regulation of the cell cycle
- Apoptosis (programmed cell death)
- Maintenance of genome stability
It is activated in response to DNA damage and pauses the cell cycle.
Let’s say if there is a DNA damage that happens during G1 phase and if these particular damages aren’t repaired these could lead to a negative consequence because these are really deleterious in terms of nature.
Basically, it blocks replication fork, loss of chromosomal segment and worst outcome will be apoptosis and death of the cell. Thus, p53 can modulate the cyclins and prevent and cause pause in the cell cycle.
Basically, there are special sensors known as ATM (Ataxia telangiectasia mutated) and ATR (Ataxia telangiectasia and Rad3-related) which can sense and bind to DNA damage. With downstream signaling pathways like chk2 (check-2), p53 can be activated. Normally, p53 is degraded but when check-2 phosphorylates p53, it is activating and activated P53 can do numerous activities. It can activate p21, a negative influencer of cyclin/CDKs. So, when P21 blocks cyclin/CDKs activities the cell cycles halt and can’t progress further.
Pausing the cell cycle is very crucial as it could give DNA repair mechanisms enough time to repair damage. There is a homologous recombination mechanism and non-homologous end joining based mechanism by which DNA can be repaired. After, the damage is repaired, cell cycle is resumed and thereby cell cycle progression happens and cell divides accordingly.
However, if the DNA damage can’t be repaired in this case the cell will be induced for apoptosis. P53 coordinated with caspases-3 will basically target the intrinsic pathway of apoptosis. It activates Bax, Bad which poke holes in the mitochondrial membrane, cytochrome-c will leak and ultimately caspase-3 is activated which triggers the responses.
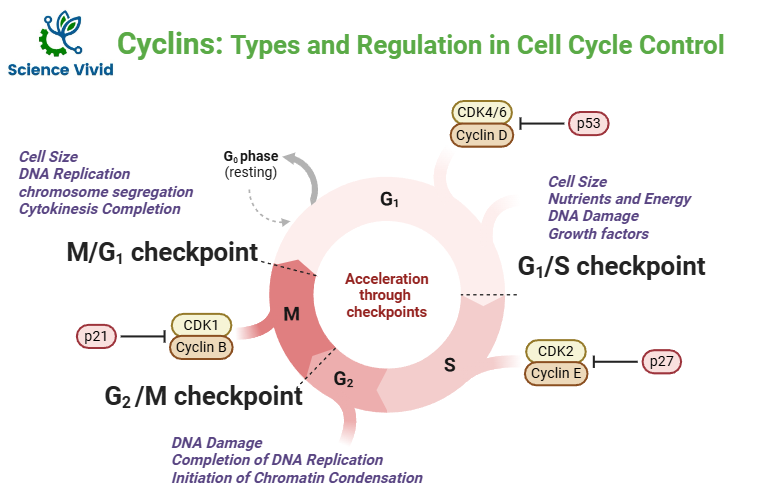
S-PHASE CYCLIN (CYCLIN E-CDK2)
The S-phase cyclin is cyclin E- CDk2 area corresponding partner.
So, at the end of G1, the replicated signal is already bound to the origin recognition complex (ORC) that binds to both Cdt1 and Cdc6 proteins. These are the crucial components of the pre-replication complex (pre-RC) that assembles at DNA replication origins during the G1 phase of the cell cycle, allowing for the initiation of DNA replication. It loads mcm2-7 helicases and this enzyme will form a replication bubble but that requires a licensing event. That licensing event is happening with the help of kinases (CDKs).
Replication initiation includes replication selection and origin activation.
In S-phase, Cyclin E-CDK2 complex is tightly act and it can actually phosphorylate both Cdt1 and Cdc6 proteins and thereby allow the replication bubble to form. It is very important to form a replication bubble at the beginning of the S-phase. And, on the other hand, since these particular Cdt1 and Cdc6 are hyper phosphorylated they can get reassembled into the replication complex. Thereby, it also ensures that replication only happens once in the cell cycle.
M-PHASE CYCLIN (CYCLIN B-CDK1)
Cyclin B-CDK1 has different phosphorylates site such as:
- Inhibitory site (Threonine 14 and Threonine 15)
- Activator site (Threonine 161)
Several kinases like wee 1 kinase phosphorylates and inhibit the activity of the cyclin B-CDK1 complex. These are also activator kinases such as CAK kinase that phosphorylate cdK1 at a specific site leading to its activation.
There are also activator phosphatases (cd25 phosphatase) that release the inhibitory phosphate group from cyclin -dependent kinase
After the activation of cyclin B-CDK1, it can lead to several biological functions.
- Chromosome condensation
- Nuclear envelope breakdown
- Fragmentations of Golgi
- Spindle formation
After the completion of the task, it will be degraded by poly-ubiquitination by the Anaphase Promoting Complex (APC). When poly-ubiquitination masks are added to the complex protease, mediated degradation will be triggered.
- Cyclin B-CDK1 is a really important kinase because in the beginning of the prophase, it phosphorylates the lamina. Lamina is an intermediate filament that supports the nuclear envelope. So, it leads to breakdown of the nuclear envelope, the nuclear gets separated and chromosomes get condensed. So, lamina dissociation is a n important event performed by cyclin B-CDK1.
- It phosphorylates condensins, a protein that aids in organizing structure of chromosomes and plays a role in gene expression. Phosphorylation of condensins by cyclin B-CDK1 promotes chromosome condensation by altering structure and activity of condensins complex, allowing them to properly package and organize genetic material before cell division.
