Introduction:
Carbohydrates are the most common organic compounds in the environment. They are essentially made up of the elements carbon, hydrogen, and oxygen. Carbohydrate literally means “carbon hydrates.”
Carbohydrates are characterized as polyhydroxy groups of aldehydes or ketones. Carbohydrates, commonly known as saccharides or carbohydrates, supply energy to the body. Four calories are contained in one gram of carbohydrate.
Carbohydrates are a type of naturally occurring organic molecules composed of carbon, hydrogen, and oxygen that are largely produced by plants. These are the ultimate source of our food. In higher animals, the simple sugar glucose is an essential component of blood and is found in polymeric form as glycogen in the liver and muscle.
Sources:
- Carbohydrates, together with proteins and lipids, are one of the three basic nutrients present in foods and beverages.
- Carbohydrates can be found in a range of foods, including bread, beans, milk, popcorn, potatoes, biscuits, spaghetti, soft drinks, maize, and cherry pie. They are also available in a variety of shapes and sizes.
- The most common and abundant types are sugars, fibres, and carbohydrates.
- They are exceedingly common in plants, accounting for up to 80% of the dry weight.
- Fructose, a simple sugar, is present in a variety of fruits.
- Galactose can be found in all dairy products.
- Lactose is widely present in milk and other dairy products.
- Maltose can be found in cereal, beer, potatoes, processed cheese, pasta, and other foods.
- The natural sources of sucrose are honey and sugar, both of which also have minute amounts of vitamins and minerals.
Functions:
Carbohydrates are a class of biomolecules that include sugars, starches, and cellulose.
- Energy production: Carbohydrates are the primary source of energy for the body. They are broken down into simple sugars, such as glucose, which can be used by cells to produce ATP, the primary energy currency of the body.
- Structural support: Some carbohydrates, such as cellulose, serve as structural components in plants, providing support and protection.
- Metabolic regulation: Carbohydrates, particularly glucose, play a role in the regulation of various metabolic processes in the body. For example, they can stimulate the release of insulin, which helps regulate blood sugar levels.
- Communication: Some carbohydrates, such as those found on the surface of cells, serve as signaling molecules that can be recognized by other cells, allowing them to communicate with each other.
- Storage: Some carbohydrates, such as glycogen, can be stored in the liver and muscles as a source of energy for later use.
Types:
Carbohydrates are broadly categorized into two types:
(i) Sugars. These are sweet and water-soluble crystalline compounds. Examples, glucose, fructose and cane sugar.
(ii) Non-sugars. They have no taste, are insoluble in water, and are amorphous. Example. Starch, cellulose, etc.
However, these days Carbohydrates are systematically classified into two major group:
On the basis of number of carbon atom
- Trioses (Three carbon atom, Eg. Glyceraldehyde)
- Tetroses (Four carbon atom, Eg. Erythrose)
- Pentoses (Five carbon atom, Eg. Ribose)
- Hexoses (Six carbon atom. Eg. Glucose, Fructose)
- Heptoses (Seven carbon atom, Eg Sedoheptulose)
On the basis of repeating units
There are three major classes of carbohydrates:
1. Monosaccharides
Monosaccharides, often known as simple sugars, are made up of a single polyhydroxy aldehyde or ketone molecule. The six-carbon sugar D-glucose, sometimes known as dextrose, is the most abundant monosaccharide in nature.
2. Oligosaccharides
Oligosaccharides are composed of short chains of monosaccharide units, or residues, connected by unique linkages known as glycosidic bonds. Disaccharides are the most prevalent, containing two monosaccharide units. Example: sucrose (cane sugar).
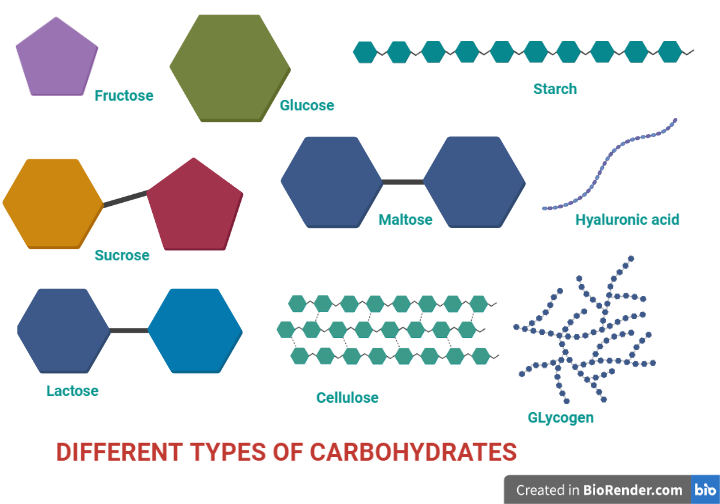
Fig: Structure of different types of carbohydrates
3. Polysaccharides
Polysaccharides are sugar polymers that contain more than 20 monosaccharide units, with some including hundreds or thousands.
It is classified further classified on the basis of their function and composition.
Based on function
A. Storage polysaccharide – starch.
B. Structural polysaccharide – cellulose.
Based on composition:
Homopolysaccharides– Homopolysaccharides (homoglycans) are composed of a single type of monomer. The best-known examples include cellulose, glycogen, and starch.
Heteropolysaccharides– Heteropolysaccharides are polysaccharides that contain different forms of monosaccharides. The best-known examples include hyaluronic acid, chitin, and heparin.
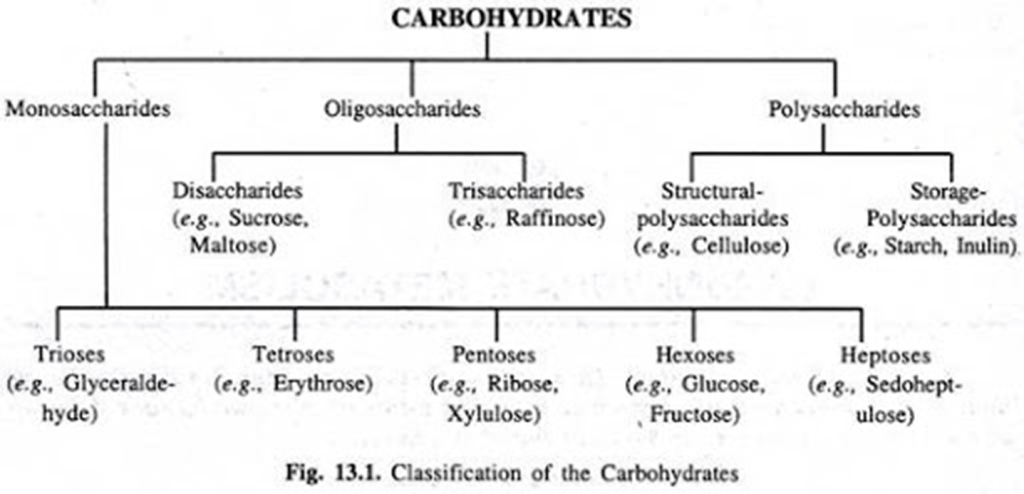
Fig: Classifications of carbohydrates
Physical Properties:
Optical activity of sugars
Compounds with asymmetric carbon atoms exhibit optical activity. When a beam of polarized light is passed through a solution of an optical isomer, it will be rotated either to the right or left. The term dextrorotatory (d+) and levorotatory (l–) are used to compounds that respectively rotate the plane of polarized light to the right or to the left.
Stereoisomerism
Compound shaving the same structural formula but they differ in spatial configuration. Example: Glucose has two isomers. They are D-glucose and L-glucose. If the hydroxyl groups of the carbon atom adjacent to alcohol are orient in the right-hand side, it is known as D-Gluocose. In contrast, if it is orient in the left-hand side, it is known as L-Gluocose.
Diastereomers
Anomers and epimers are both diastereomers.
If the structural orientation is different with respect to anomeric carbon or first carbon, it is known as anomers. E.g., Alpha glucose and Beta glucose.
Whereas, if there is difference in the structural orientation in carbon atoms other than anomeric carbon or first carbon, it is known as epimer. Glucose and mannose are an example of epimer with respect to carbon 2. Gluocose and galactose are an example of epimer with respect to carbon 4.
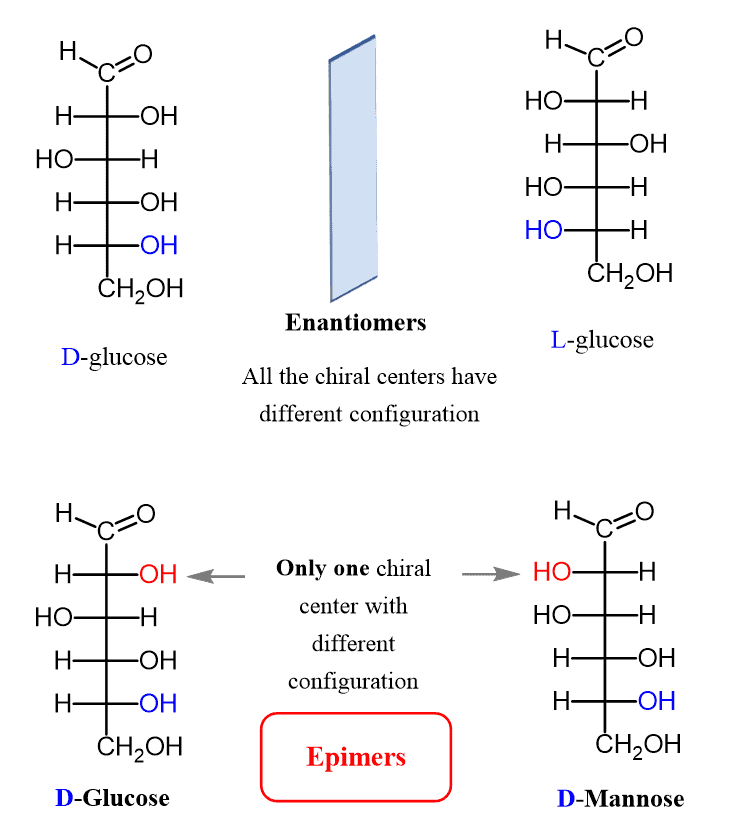
Fig: D-Glucose-and-L-Glucose-are-enantiomers-while-D-Glucose-and-D-mannose-are-epimers
Chemical Properties:
Reducing properties
The reducing property of anomeric carbon is due to the free aldehyde or keto group.
Many tests are used in the laboratory to determine carbohydrates’ reducing action. Benedict’s test, Fehling’s test, Barfoed’s test, and others are examples. Sucrose is a non-reducing sugar.
Oxidation:
The terminal aldehyde (or keto) or terminal alcohol, or both groups, may be oxidized depending on the oxidizing agent used.
1. Oxidation of aldehyde group (CHO to COOH) results in the formation of gluconic acid.
2. Oxidation of terminal alcohol group (CH2OH to COOH) leads to the production of
glucuronic acid.
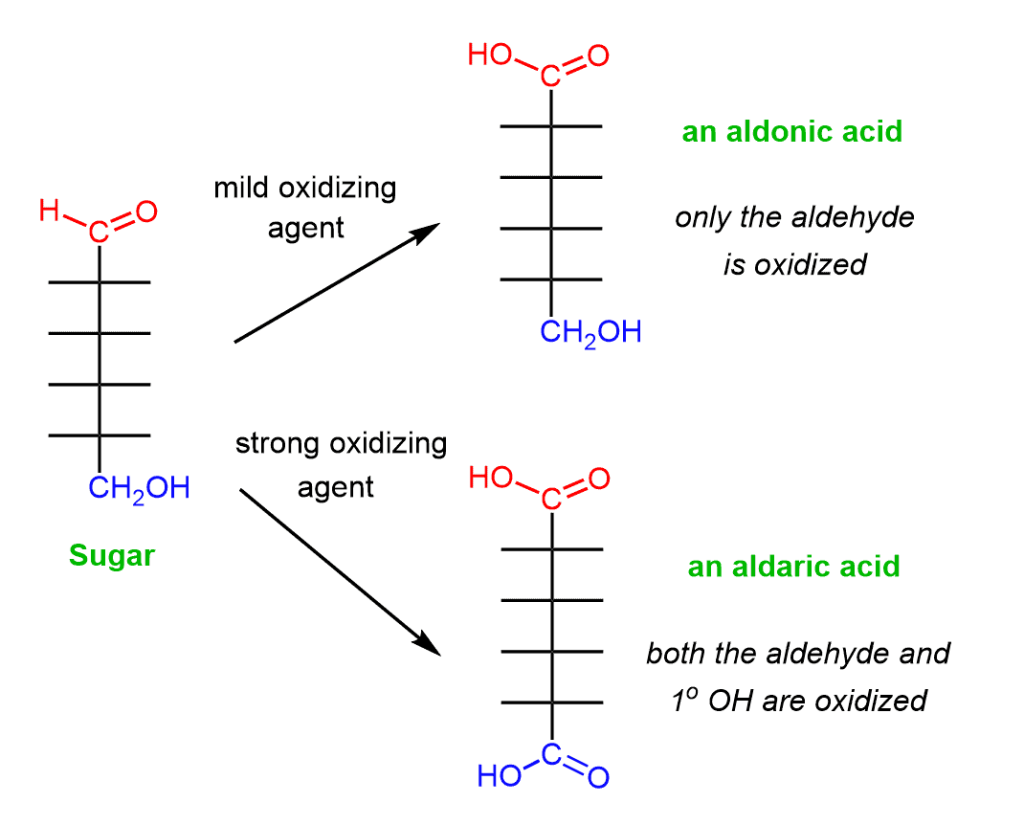
Fig: Oxidation of carbohydrates
Reduction
The aldehyde or keto group of a monosaccharide is converted to equivalent alcohol when handled with reducing agents such as sodium amalgam.
For example, glucose is changed to sorbitol, mannose to mannitol, and so on.
Dehydration
Monosaccharides dehydrate when exposed to strong sulfuric acid, with the loss of three water molecules. On dehydration, hexoses give hydroxymethyl furfural, whereas pentoses give furfural.
This is the principle of Molisch test which is designed to detect the presence of carbohydrate molecules in a specific test sample.
References:
- Jequier, E., 1994. Carbohydrates as a source of energy. The American journal of clinical nutrition, 59(3), pp.682S-685S.
- Madigan, M. T., Martinko, J. M., Bender, K. S., Buckley, D. H., & Stahl, D. A. (2015). Brock biology of microorganisms (Fourteenth edition.). Boston: Pearson.
- David, L., Nelson, D.L., Cox, M.M., Stiedemann, L., McGlynn Jr, M.E. and Fay, M.R., 2000. Lehninger principles of biochemistry.
- Satyanarayana, U., 2021. Biochemistry, 6e-E-book. Elsevier Health Sciences.
- Voet, D. and Voet, J.G., 2010. Biochemistry. John Wiley & Sons.
- Holesh, J.E., Aslam, S. and Martin, A., 2017. Physiology, Carbohydrates.
