Introduction:
It is an analytical technique used to determine the concentration of certain elements, metal atoms/ions in given sample. Metals which consist of 75% of earth chemical elements sometime also make a content as a contaminant in several components (mine. Foods, water, etc)
Therefore, this technique uses the principle in which atoms (and ions) can absorb light at a specific, unique wavelength and the energy (photon of light) is absorbed by the atom. Eventually, electrons will be excited and it will move from the ground state to an excited state. Subsequently, the amount of light absorbed is measured and the concentration of the element in the sample can be calculated.
Principle:
The sample is typically in liquid form, and after the vaporisation steps the target elements, it is converted to free atoms through introduction into a flame through nebuliser. The principle is based on the absorption of photon of light by free, ground-state atoms in the gaseous state.
A lamp source emit light at wavelength specific to the atoms which is in gaseous state through the flame. As, the light energy is absorbed, the electrons in the atoms are elevated to an excited state by absorbing energy (photon of light)
In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly.
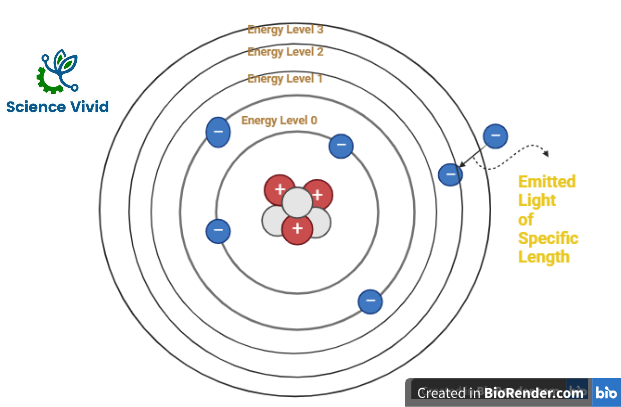
Fig: Principle of Atomic Absorption Spectroscopy (AAS)
Instrumentation:
- A sample introduction system
- The burner (flame) and its associated gas supplies: air-acetylene or nitrous oxide-acetylene
- A light source, the Hollow Cathode Lamp (HCL)
- A monochromator (the optical components inside the box in the diagram)
- An optical detector (photomultiplier tube or PMT)
- Computerized instrument control, data collection record, and analysis.
Light Source
The light source is one of the critical component and Hollow Cathode Lamp (HCL) is the most common light source used with an AAS. It consists of a same element of which its concentration needs to be analysed in the analyte. The lamp is filled with an inert ‘filler’ gas usually argon or neon at low pressure.
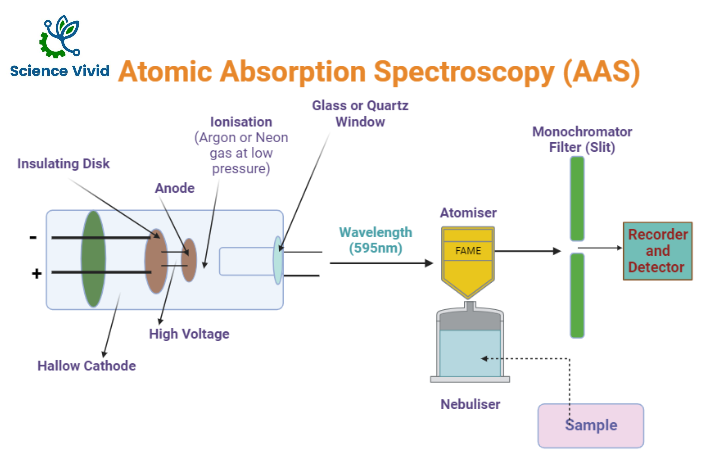
Fig: Instrumentation of Atomic Absorption Spectroscopy (AAS)
The filler gas will be accelerated towards the cathode after the application of high voltage across the two electrodes. Furthermore, collisions between metal atoms occurs raising them to an excitation state and after the atoms retuned t its ground state, radiation with specific wavelength is emitted. It is the absorbance of the light from the lamp as it passes through the atomized sample, that is measured.
The monochromator
The light emitted by hollow cathode lamp is passed through a monochromator. It is an optical device that is used to select light of narrow range from a broader spectrum of light which is emitted from a light source.
Sample introduction system
The liquid sample is introduced at a higher velocity into the nebulizer via a narrower capillary tube.
A fine spray of droplets will be created after high velocity fluid sample will impacts a glass bead. A fine spray of droplets is known as aerosol. However, larger droplets drain out as a waste to maintain a homogenous flow of fine droplets into the spray chamber, while the fine aerosol is passed up into the spray chamber.
There are several techniques of atomiser.
Flame Atomic Absorption Spectroscopy (FAAS)
After nebulisation, the metal ions are finely spray into a high-temperature flame where it is reduced to their atoms and subsequently absorb light from an element-specific hollow cathode lamp.
- Desolvation, or drying. The solvent is evaporated, resulting in dry nanoparticles of the sample remaining.
- Vaporization. The particles are converted to the gaseous phase
- Atomization. The key stage at which the population of ground state, freely dissociated atoms is created. Ground state atoms are the target for AAS analysis.
- Ionization. Some free atoms will be converted to ions which will depend on the flame conditions (mixture of the oxidant/acetylene gases mix) and the ionization potential of the analytes on solution.
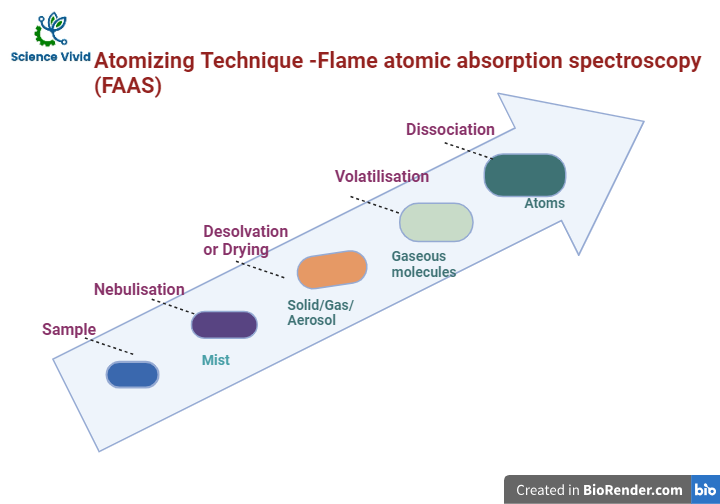
Fig: Flame Atomic Absorption Spectroscopy (FAAS)
Graphite furnace atomic absorption spectroscopy (GFAAS)
It is a type of electrothermal atomization where a sample is placed in a hollow graphite tube The unit is heated until the sample is completely vaporized.
The detector
The photomultiplier tube (PMT) is one of the mostly used detector in atomic absorption spectrometer because of its high sensitivity.
The incident light from the exit slit of the monochromator enter the PMT and hit a photodiode which is eventually converted to an electrical signal. The primary electrical signal will be amplified by a series of dynodes and it is collected
A series of dynodes amplify the signal by 100 million times and then it is collected (measured) which is used to provide a quantitative measurement of absorption.
Applications:
- It is used in order to identify the presence of the heavy metals (toxic form) such as palladium or platinum in the antibiotics.
- The food, health supplement industries, and cannabis make use of AAS to ensure that if there is any contamination in the supplied products and make sure they are safe for consumption.
- In mining, the amount of gold can be quantified using AAS.
- AAS is mostly used in the analysis of drinking water in the most polluted areas.
