Introduction:
An antibody is a protein produced by the immune system that is specifically designed to recognize and bind to a specific target molecule, known as an antigen.
Antibodies are produced by a type of immune cell called B cells, and are also known as immunoglobulins.
Antibodies are an important part of the immune system, and play a key role in protecting the body against infections and other diseases.
They work by recognizing and binding to specific molecules on the surface of pathogens, such as viruses or bacteria, or to molecules that are produced by the body in response to an infection or other immune trigger. This binding can help to neutralize the pathogen, mark it for destruction by other immune cells, or trigger other immune responses.
Features:
They have several important characteristics that contribute to their function in protecting the body from disease:
Specificity: Antibodies are highly specific and are able to recognize and bind to a particular foreign substance, such as a particular type of bacteria or virus.
Diversity: There are many different types of antibodies, each with a unique structure and specific target. This diversity allows the immune system to mount a broad response to a wide range of foreign substances.
Affinity: Antibodies have a high affinity for their specific target, meaning that they can bind to it very tightly. This helps to ensure that the foreign substance is effectively neutralized and eliminated from the body.
Structural diversity: Antibodies have a highly variable structure, with the specific structure of an antibody determining its target and function.
Solubility: Antibodies are soluble proteins, meaning that they can dissolve in water and other fluids. This allows them to circulate throughout the body and reach the site of an infection or other foreign substance.
Structure:
Antibodies are proteins that are composed of four polypeptide chains: two heavy chains and two light chains. The heavy chains are responsible for determining the specific antigen that the antibody can bind to, while the light chains contribute to the overall shape of the antibody.
Moreover, the structure of an antibody is highly variable, with the variable region responsible for recognizing and binding to specific antigens, while the constant region is responsible for the function of the antibody.
The structure of an antibody is characterized by several different regions, including the following:
Variable region
This is the part of the antibody that is responsible for recognizing and binding to specific antigens. The variable region is highly variable and is specific to each individual antibody.
Constant region
This is the part of the antibody that is responsible for its function and is not as variable as the variable region. The constant region determines the class of the antibody (IgM, IgG, IgA, IgD, or IgE) and is the same for all antibodies within a given class.
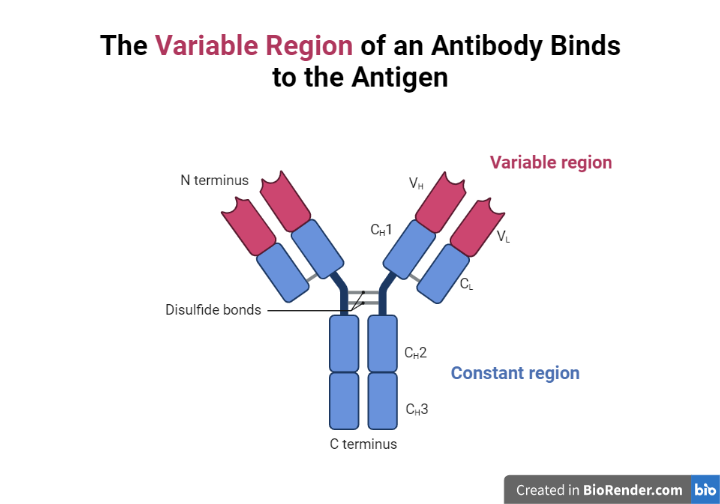
Fig: Structure of antibodies
Fc region
This is the part of the antibody that is responsible for activating other immune cells, such as phagocytes and killer T cells. The Fc region is located in the constant region of the heavy chain.
Fab region
This is the part of the antibody that contains the variable and constant regions of both the heavy and light chains and is responsible for binding to antigens. The Fab region is located at the ends of the heavy and light chains.
Types:
There are five main types of antibodies, also known as immunoglobulins, based on the structure of their heavy chains. Each type of antibody has a specific function and is produced in response to different types of antigens. The body produces a large and diverse repertoire of antibodies in response to different infections and other immune triggers, and each individual typically produces a unique set of antibodies in their lifetime.
IgM: This is the first type of antibody produced in response to an infection or other immune trigger. IgM antibodies are large molecules that are found in the blood and other bodily fluids. They are effective at neutralizing pathogens and activating other immune cells.
IgG: This is the most abundant type of antibody in the body, and is found in all body fluids. IgG antibodies are produced in response to an infection or other immune trigger and are effective at neutralizing pathogens and activating other immune cells.
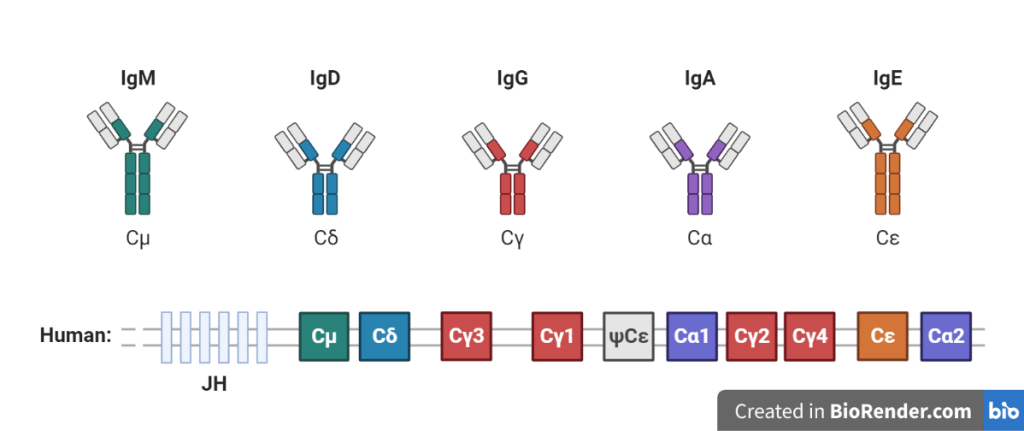
Fig: Different types of antibodies
IgA: This type of antibody is found in mucosal surfaces, such as the lining of the respiratory and gastrointestinal tracts. IgA antibodies help to protect these surfaces from infections.
IgD: This type of antibody is found on the surface of B cells and plays a role in the activation of these cells.
IgE: This type of antibody is involved in allergic reactions and is produced in response to allergens. IgE antibodies bind to mast cells and basophils, triggering the release of inflammatory chemicals.
Production And Mechanism of Antibody
- The production of antibodies is a complex process that involves several different types of immune cells, including B cells and T cells. When a foreign substance enters the body, the immune system identifies it as a potential threat and begins to produce antibodies to target and neutralize it.
- B lymphocytes, or B cells, are a subtype of white blood cell that produces antibodies. When a B cell encounters a foreign substance, it becomes activated and begins to divide and produce a large number of identical copies of itself, called clones. Each clone produces a specific type of antibody that is able to recognize and bind to a particular foreign substance, such as a particular type of bacteria or virus.
- T cells, also known as T lymphocytes, are another type of white blood cell that play a crucial role in the immune response. T cells help to activate B cells and other immune cells, as well as providing additional support and protection against infection.
- The mechanism of antibody production involves the activation of B cells and T cells, the proliferation and differentiation of B cells into plasma cells, and the synthesis and secretion of antibodies by plasma cells. When a foreign substance enters the body, the immune system recognizes it as a threat and activates B cells and T cells to begin the process of producing antibodies.
- B cells then divide and differentiate into plasma cells, which are specialized cells that produce large amounts of a specific type of antibody. The antibodies are then secreted into the bloodstream, where they circulate and bind to the foreign substance, neutralizing it and helping to protect the body from infection.
Polyclonal and monoclonal antibody:
Polyclonal antibodies are antibodies that are produced by multiple different B cells in response to a particular foreign substance, such as a virus or bacteria. These antibodies are typically produced in response to a natural infection or immunization and are made up of a diverse range of different antibodies, each with a unique structure and specific target.
Monoclonal antibodies, on the other hand, are produced by a single clone of B cells and are identical in structure and function. They are produced in a laboratory setting using specialized techniques, such as hybridoma technology, which involves the fusion of a B cell with a cancerous cell to create a stable cell line that can produce a specific monoclonal antibody.
Polyclonal antibodies are typically used in research settings to detect the presence of a specific protein or antigen in a sample. They are also used in diagnostic tests and as therapeutics to stimulate the immune system and help the body fight infection. Monoclonal antibodies, on the other hand, are more commonly used as therapeutics to specifically target and neutralize a particular protein or antigen in the body. They are used in the treatment of a variety of diseases, including cancer, autoimmune disorders, and infectious diseases.

Fig: Polyclonal and monoclonal antibody
There are several key differences between polyclonal and monoclonal antibodies:
Production: Polyclonal antibodies are produced naturally in response to an infection or immunization, while monoclonal antibodies are produced artificially in the laboratory using hybridoma technology.
Diversity: Polyclonal antibodies are made up of a diverse range of different antibodies, each with a unique structure and target, while monoclonal antibodies are all identical in structure and function.
Specificity: Polyclonal antibodies may recognize and bind to multiple different targets, while monoclonal antibodies are highly specific and only recognize and bind to a single target.
Applications: Polyclonal antibodies are typically used in research settings to detect the presence of a specific protein or antigen in a sample. They are also used in diagnostic tests and as therapeutics to stimulate the immune system and help the body fight infection. Monoclonal antibodies, on the other hand, are more commonly used as therapeutics to specifically target and neutralize a particular protein or antigen in the body. They are used in the treatment of a variety of diseases, including cancer, autoimmune disorders, and infectious diseases.
Functions:
Antibody have a significant impact on the body’s ability to fight disease and perform a number of critical tasks, such as:
Neutralization: Antibodies can bind to and neutralize viruses and toxins, helping to protect the body from infection and disease.
Opsonization: Antibodies can coat foreign substances, such as bacteria and viruses, making them more visible to the immune system and easier for immune cells to detect and destroy.
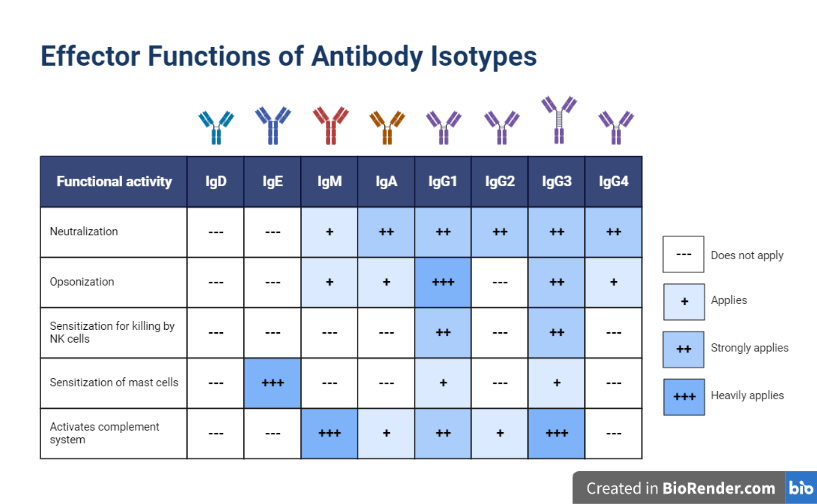
Fig: Effector Functions of Antibody Isotypes
Activation of the complement system: Antibodies can activate the complement system, which is a group of proteins that work together to attack and destroy foreign substances.
Antigen presentation: Antibodies can help to present antigens (foreign substances) to immune cells, activating the immune response and helping the body to recognize and target specific pathogens.
Allergy prevention: Antibodies can help to prevent allergic reactions by binding to and neutralizing allergens (substances that cause allergic reactions).
References:
- Owen, J.A., Punt, J. and Stranford, S.A., 2013. Kuby immunology (p. 574). New York: WH Freeman.
- Bellanti, J. ed., 2013. Immunology (Vol. 6). Springer Science & Business Media.
- Male, D.K., 2006. Immunology. Elsevier Health Sciences.

