Introduction:
Allosteric regulation refers to the process by which the activity of an enzyme or protein is regulated by the binding of a molecule to an allosteric site on the protein, which is distinct from the protein’s active site. This binding event can cause a conformational change in the protein, altering its activity or affinity for other molecules.
Allosteric regulation is a critical mechanism for controlling enzyme activity and regulating cellular processes. It allows cells to respond to changes in their environment and adjust their metabolic processes accordingly. Allosteric regulation can act as a positive or negative feedback mechanism, depending on the nature of the allosteric molecule and its effect on protein activity.
Allosteric regulation is often used in metabolic pathways to maintain homeostasis and prevent the overproduction or depletion of essential metabolites. For example, the enzyme phosphofructokinase (PFK) is regulated by allosteric binding of ATP and ADP, which can indicate the cellular energy status and adjust the rate of glycolysis accordingly.
In addition to enzymes, allosteric regulation can also occur in other types of proteins, such as ion channels and receptors. For example, neurotransmitter receptors can be allosterically regulated by ligands that bind to allosteric sites on the receptor, altering its conformation and activity.
Principle:
The principle of allosteric regulation is based on the ability of certain molecules to bind to specific sites on a protein, known as allosteric sites, that are distinct from the protein’s active site. Binding of these molecules to the allosteric site induces a conformational change in the protein, which alters its activity or affinity for other molecules.
In positive allosteric regulation, the binding of an allosteric activator enhances the protein’s activity or affinity for other molecules, while in negative allosteric regulation, the binding of an allosteric inhibitor reduces the protein’s activity or affinity for other molecules.
Allosteric regulation allows cells to respond to changes in their environment by modulating the activity of enzymes and proteins. It can also allow for fine-tuning of biochemical pathways by regulating key enzymes, and for the creation of more specific and targeted drug therapies by selectively modulating the activity of enzymes and receptors.
Types:
There are two main types of allosteric regulation: positive allosteric regulation and negative allosteric regulation.
Positive allosteric regulation
In positive allosteric regulation, the binding of an allosteric molecule to the protein enhances its activity or affinity for other molecules. This can be achieved through a conformational change in the protein that enhances the protein’s ability to bind to other molecules. For example, the binding of oxygen to hemoglobin induces a conformational change that enhances the protein’s affinity for additional oxygen molecules.
Properties
Positive allosteric regulation is a process by which the binding of a molecule to an allosteric site on a protein enhances the protein’s activity or affinity for other molecules. Some properties of positive allosteric regulation include:
Enhances enzyme activity: Positive allosteric regulation can enhance the activity of enzymes involved in metabolic pathways. This allows cells to respond to changes in their environment and adjust their metabolic processes accordingly.
Non-competitive binding: The molecule that binds to the allosteric site does not compete with the substrate for the active site. Instead, it induces a conformational change in the protein that enhances its ability to bind to the substrate.
Cooperative binding: Positive allosteric regulation often involves cooperative binding, where the binding of one molecule to the allosteric site enhances the affinity of the protein for additional molecules. This can result in a sigmoidal binding curve, where the binding affinity of the protein increases rapidly as more molecules bind.
Amplifies cellular response: Positive allosteric regulation can amplify cellular responses to external stimuli. For example, the binding of oxygen to hemoglobin in the lungs induces a conformational change that enhances the protein’s affinity for additional oxygen molecules, allowing for efficient oxygen transport in the blood.
Can act as a switch: Positive allosteric regulation can act as a molecular switch to turn on or off specific cellular pathways. For example, the binding of cyclic AMP (cAMP) to the regulatory subunit of protein kinase A (PKA) induces a conformational change that releases the catalytic subunits, activating the enzyme and initiating downstream signaling pathways.
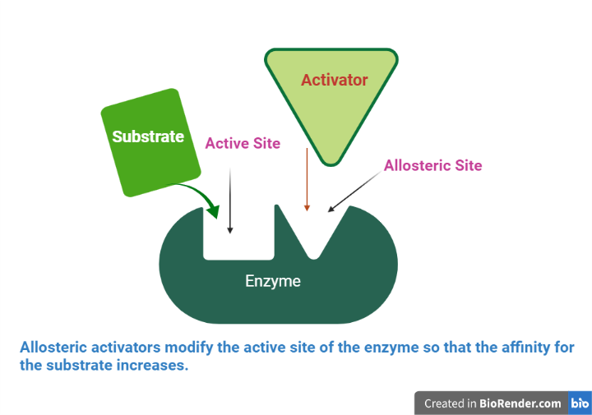
Fig: Allosteric activators modify the active site of the enzyme so that the affinity for the substrate increases.
Examples
Cyclic AMP (cAMP): cAMP is a small molecule that acts as a positive allosteric regulator of protein kinase A (PKA). Binding of cAMP to the regulatory subunit of PKA induces a conformational change that releases the catalytic subunit, activating the enzyme and initiating downstream signaling pathways.
ATP: ATP acts as a positive allosteric regulator of pyruvate kinase, a key enzyme in glycolysis. Binding of ATP to an allosteric site on pyruvate kinase stabilizes the enzyme’s inactive conformation, reducing its activity.
Citrate: Citrate is a positive allosteric regulator of acetyl-CoA carboxylase, an enzyme involved in fatty acid biosynthesis. Binding of citrate to an allosteric site on acetyl-CoA carboxylase enhances the enzyme’s activity, increasing the production of fatty acids.
2,3-bisphosphoglycerate (2,3-BPG): 2,3-BPG is a positive allosteric regulator of hemoglobin, an oxygen-binding protein in red blood cells. Binding of 2,3-BPG to an allosteric site on hemoglobin induces a conformational change that reduces the protein’s affinity for oxygen, allowing for efficient oxygen delivery to tissues.
Calcium ions: Calcium ions act as positive allosteric regulators of calmodulin, a protein involved in signal transduction pathways. Binding of calcium ions to calmodulin induces a conformational change that allows it to bind to target proteins, initiating downstream signaling events.
Negative allosteric regulation
In negative allosteric regulation, the binding of an allosteric molecule to the protein reduces its activity or affinity for other molecules. This can be achieved through a conformational change in the protein that decreases its ability to bind to other molecules.
Allosteric inhibition is a type of enzyme inhibition where the inhibitor molecule binds to a site on the enzyme molecule that is different from the active site, known as the allosteric site. This binding causes a conformational change in the enzyme molecule that alters the shape of the active site, making it less accessible to the substrate and thereby inhibiting the enzyme’s activity.
Allosteric inhibition can be either reversible or irreversible, depending on the strength of the binding between the inhibitor molecule and the allosteric site. Reversible inhibition occurs when the inhibitor molecule can bind and dissociate from the allosteric site, allowing the enzyme to return to its active state once the inhibitor is removed. Irreversible inhibition occurs when the inhibitor molecule forms a covalent bond with the allosteric site, permanently altering the enzyme’s structure and function.
Properties
Non-competitive binding: Allosteric inhibitors bind to a site on the enzyme molecule that is different from the active site, known as the allosteric site. This binding does not compete with the substrate for binding to the active site of the enzyme.
Conformational change: Binding of the allosteric inhibitor to the allosteric site induces a conformational change in the enzyme molecule, altering the shape of the active site and reducing its affinity for the substrate.
Feedback inhibition: Allosteric inhibition can act as a feedback mechanism to regulate enzyme activity. For example, ATP can act as an allosteric inhibitor of phosphofructokinase in the glycolytic pathway, slowing down the pathway when there is already an abundant supply of ATP.
Cooperativity: Allosteric inhibition can act cooperatively with other regulatory molecules to fine-tune enzyme activity in response to changing cellular conditions. For example, in the regulation of hemoglobin, the binding of oxygen to one subunit of the protein enhances the binding of oxygen to other subunits, while the binding of allosteric inhibitors, such as 2,3-bisphosphoglycerate, can inhibit the binding of oxygen and promote the release of oxygen from the protein.
Reversibility: Allosteric inhibition can be reversible or irreversible, depending on the strength of the binding between the allosteric inhibitor and the allosteric site. Reversible inhibitors can bind and dissociate from the allosteric site, while irreversible inhibitors form covalent bonds with the enzyme and permanently alter its structure and function.

Fig: Allosteric inhibitors modify the active site of the enzyme so that substrate binding is reduced or prevented.
Example of allosteric inhibitor
One example of an allosteric inhibitor is ATP (adenosine triphosphate) in the enzyme phosphofructokinase (PFK), which is involved in the glycolytic pathway. ATP binds to the allosteric site of PFK and inhibits its activity by causing a conformational change that reduces the enzyme’s affinity for its substrate, fructose-6-phosphate. This feedback mechanism helps regulate the rate of glycolysis by slowing down the pathway when there is already an abundant supply of ATP, conserving energy for future use.
Another example of an allosteric inhibitor is the drug methotrexate, which is used as a chemotherapy agent to treat cancer. Methotrexate inhibits the enzyme dihydrofolate reductase, which is involved in the synthesis of nucleotides and is necessary for DNA replication. Methotrexate binds to the allosteric site of dihydrofolate reductase and inhibits its activity, preventing the synthesis of nucleotides and slowing down the growth of cancer cells.
Models:
Allosteric regulation is a type of protein regulation where a molecule binds to a protein at a site other than the protein’s active site, causing a conformational change that alters the protein’s activity. There are two main models of allosteric regulation:
The concerted model (also known as the MWC model)
This model proposes that all subunits of a protein with multiple subunits exist in either a relaxed (R) state or a tense (T) state. The binding of an allosteric effector to one subunit of the protein can induce a conformational change in the entire protein, shifting all subunits into either the R state or the T state. This model is named for its proposal that all subunits of the protein work together in a concerted manner to undergo the conformational change.
The sequential model
This model proposes that subunits of a protein with multiple subunits can exist in intermediate states between the R and T states. The binding of an allosteric effector to one subunit can induce a conformational change in that subunit, which can then affect the conformation of adjacent subunits. This model suggests that subunits can be individually affected and the conformational changes propagate through the protein sequentially.
Applications:
Drug discovery: Allosteric inhibitors and activators are attractive targets for drug discovery because they can modulate the activity of enzymes and receptors without directly interfering with their active sites. This allows for more specific and targeted modulation of biological pathways, with potentially fewer side effects. For example, the drug ibuprofen is an allosteric inhibitor of the enzyme cyclooxygenase, which is involved in inflammation and pain.
Enzyme engineering: Allosteric regulation can be engineered into enzymes to create novel properties or to enhance their catalytic activity. For example, the enzyme aspartate transcarbamoylase (ATCase) is naturally regulated by feedback inhibition by its product, CTP. By engineering ATCase to be regulated by a different molecule, researchers can create an enzyme that is activated or inhibited by a specific molecule, potentially for use in industrial or biotechnological applications.
Biotechnology: Allosteric regulation is used in various biotechnological applications, including biosensors and protein engineering. Biosensors use allosteric proteins to detect specific molecules or ions, while protein engineering uses allosteric regulation to create proteins with new functions or improved stability.
Agriculture: Allosteric regulation is used in plant breeding to enhance crop productivity and stress tolerance. For example, the enzyme pyruvate kinase is involved in the production of starch in plants and can be engineered to be allosterically regulated by small molecules, allowing for more efficient starch production.
References:
- Monod, J., Wyman, J., and Changeux, J. P. (1965). On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 12: 88-118.
- Koshland, D. E., Némethy, G., and Filmer, D. (1966). Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry 5: 365-385.
- Hill, A. V. (1910). The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 40: iv-vii, 1-4.
- Perutz, M. F. (1970). Stereochemistry of cooperative effects in haemoglobin. Nature 228: 726-739.
- Changeux, J. P., Kasai, M., Lee, C. Y., and Usechak, P. (1970). Allosteric transitions of the acetylcholine receptor. Prog. Biophys. Mol. Biol. 21: 197-246.
