Stages in the Formation of B Lymphocytes:
- The formation of B lymphocytes occurs in three stages:
- Primary lymphoid organs produce mature, immunocompetent B cells.
- When mature B cells come into contact with antigens in secondary lymphoid organs, they become activated.
- Once activated, B cells undergo transformation within secondary lymphoid organs, evolving into memory cells for long-term immunity and plasma cells that secrete antibodies to combat pathogens.
From bone marrow progenitors, mature antigen-responsive B lymphocytes proliferate and settle in peripheral lymphoid tissues, where they engage in interactions with foreign antigens.
The binding of antigen to membrane Ig molecules, which together with the related Igα and Igβ chains form the antigen receptor complex (BCR) of mature B cells, starts the activation of antigen-specific B lymphocytes.
Thymus-dependent (TD) Antigens- induced B-cell Activation:
Direct interaction with helper T (TH) cells is necessary for the B-cell response to thymus-dependent (TD) antigens.
Antigen Processing and Presentation
Activation of antigen-specific B lymphocytes begins when the antigen binds to membrane-bound immunoglobulin (Ig) molecules—components of the antigen receptor complex on mature B cells—along with associated Igα and Igβ proteins.
B and T Cell Interaction
This ties the activation process together. After internalizing the associated antigen into endosomal vesicles, the receptor breaks it down into peptides that may be seen on the surface of B cells for helper T cells to recognize if the antigen is a protein.
When antigen activates B cells, class II major histocompatibility complex (MHC) molecules and B7 costimulators are expressed at higher levels. Affinity maturation and heavy chain isotype flipping are commonly observed in humoral immune responses to protein antigens that are dependent on helper T cells.
Sequence of Events during T Cell–Dependent Antibody Responses:
To initiate T cell–dependent antibody responses, antigens must first be captured and transported to the B cell zones within lymphoid tissues. Mature B cells actively search for their particular antigen targets as they move through secondary lymphoid organs. Follicle B cells, which make up the majority of B cells, enter follicles. Before coming into contact with an antigen, naive follicular B cells have a short lifespan. Antigen, which might be soluble or presented by other cells, activates B cells in the lymphoid follicles. After processing and presenting the antigen, B cells move in the direction of the T cell zone and change the profile of their cell surface chemokine receptors.
CD40L and chemokine receptors are expressed by activated helper T cells, which facilitate their migration along a chemokine gradient in the direction of the follicle. At the border between the T cell zone and follicular, activated helper T cells and B cells interact. Here, the B cells are activated by the cytokines released by the T cells and the CD40L of the helper T cells.
B-cell and T-cell interaction and activation involve:
The T-B conjugate is formed
The antigen is absorbed by receptor-mediated endocytosis and converted into peptides via the endocytic pathway following its binding by mIg on B cells. Class II MHC molecules and the co-stimulatory ligand B7 are among the cell-membrane molecules that the B cell upregulates as a result of antigen binding, which also starts signaling through the BCR. The B cell’s capacity to act as an antigen-presenting cell in TH-cell activation is improved by increased production of both of these membrane proteins. A class II MHC protein on a B cell’s membrane displays a processed antigenic peptide that a TH cell recognizes. The two cells then combine to form a T-B conjugation and release cytokines directed at the antigen-specific B cell.
Contact-dependent help is mediated by CD40/CD40L interaction
When a T-B conjugate is formed, CD40L is up-regulated in addition to TH-cell cytokines being released in a specific direction. The B cell receives a signal from the interaction of CD40L with CD40 on the B cell, which, when combined with the signal produced by mIg crosslinkage, propels the B cell into G1. Numerous intracellular signaling pathways transduce the signals from CD40, which ultimately alters gene expression.
Signals provided by TH-cell cytokines
Membrane proteins from activated TH cells can urge B cells to multiply, but without cytokines, they are unable to differentiate. The polarized release of cytokines toward the interacting B cell is triggered by the antigen-specific contact between a TH and a B cell.
When the B cell is activated, it starts to express membrane receptors for different cytokines, including IL-2, IL-4, and IL-5. The cytokines that the contacting TH cell produces are subsequently bound by these receptors. These cytokine-receptor interactions generate signals that promote B-cell proliferation and can trigger affinity maturation, class switching, and differentiation into memory B cells and plasma cells.
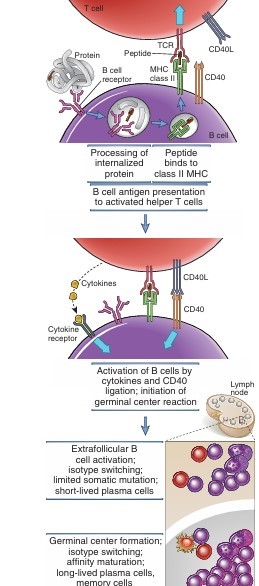
Fig: Mechanisms of helper T cell–mediated B cell activation
Source: Abbas, A. K., Lichtman, A. H., & Pillai, S. (2012). Cellular and molecular immunology. 7th ed. Elsevier/Saunders.
Thymus Independent (TI) B cell activation:
Antigens that can activate B cells without needing to come into direct touch with helper T (TH) cells are known as thymus-independent (TI) antigens. Based on how they work, they are divided into two types: TI-1 and TI-2. Regardless of their antigen specificity, B lymphocytes can be stimulated by TI-1 antigens, such as lipopolysaccharide (LPS), which is present in bacterial cell walls.
Many TI-1 antigens function as mitogens, inducing widespread, polyclonal activation of B cells.
On the other hand, TI-2 antigens—like bacterial polysaccharides with repeated sugar units or polymeric proteins such as flagellin—are highly repetitive in structure. At low concentrations, TI-2 antigens prompt the production of LPS-specific antibodies, while at higher doses, they also function as polyclonal activators of B cells.
While TH cells are not directly involved in the B-cell response to TI-2 antigens, TH cell-derived cytokines are necessary for effective B-cell proliferation and class flipping to isotypes other than IgM. The practical significance of TI antigens is that humoral immunity is the main defense mechanism of the host against infections by such encapsulated bacteria, and many bacterial cell wall polysaccharides fall into this group. Because of this, people who have inherited or acquired humoral immune impairments are particularly vulnerable to potentially fatal infections caused by encapsulated bacteria such Haemophilus, Meningococcus, and Pneumococcus.
References:
- Abbas, A. K., Lichtman, A. H., & Pillai, S. (2012). Cellular and molecular immunology. 7th ed. Elsevier/Saunders.
