Introduction:
Brain, eye and kidney are the form of organs that have tissues that use glucose as their primary or only source of metabolic fuel. Glycogen stores become exhausted during a continuous fast or intensive exercise, and glucose must be synthesized from scratch to maintain the glucose levels in the blood.
The metabolic process through which glucose is produced from non-carbohydrate metabolites in living organisms is called gluconeogenesis. Pyruvate, lactate, a few gluconeogenic amino acids, and glycerol, which is mostly produced by the metabolism of fat, are the major precursors of gluconeogenesis.
It is described as the de novo synthesis of glucose from non-hexose substrates. The production of glucose from glycogen through glycogenolysis or the liver’s conversion of fructose or galactose into glucose are not included in the process of “gluconeogenesis.”
During fasting, this ubiquitous process supplies glucose to the body when it is not obtained from food. It is almost a reversal of glycolysis. The generation of glucose is extremely important for the organs and cells that cannot use fat as fuel.
Location:
The metabolic processes of glucose synthesis and glycolysis are nearly reversible. Glycolysis is effectively reversed during glucose synthesis. However, gluconeogenesis uses four distinct enzymes to get around the three highly exergonic steps of glycolysis. The pyruvate carboxylase, PEP carboxykinase, fructose 1,6-bisphosphatase, and glucose 6-phosphatase enzymes are specific to gluconeogenesis. Gluconeogenesis can only take place in particular tissues because these enzymes are not found in all cell types. The liver and renal cortex, as well as the small intestine to a lesser extent, are where the gluconeogenesis pathway is primarily found.
Precursors/Substrates:
Lactate, pyruvate, propionate, glycerol, and 18 of the 20 (the exceptions are leucine and lysine) amino acids are some of the primary substrates for gluconeogenesis (the exceptions are leucine and lysine). Fatty acids cannot be turned into glucose because they produce acetyl coenzyme A (CoA), which enters the citric acid cycle and is oxidized to carbon dioxide (CO2).
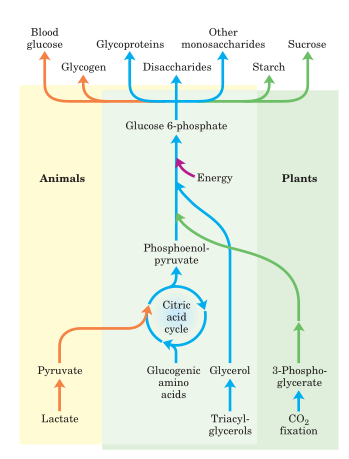
Fig: Carbohydrate synthesis from simple precursors.
Propionate, a three-carbon fatty acid, is an exception because it is carboxylated, transformed to succinyl-CoA instead of acetyl CoA, and enters the citric acid cycle as a four-carbon intermediate. Acetone, which can be converted into propanediol, is a very small gluconeogenic precursor.
Additionally, during -oxidation, the final three carbon atoms of odd-chain fatty acids produce proprionyl CoA, making them partially gluconeogenic.
Reactions of gluconeogenesis:
The pathway of gluconeogenesis is simply a reversal of glycolysis, which is responsible for the breakdown of glucose. However, only there is a difference in major enzymes between them. They are:
In the tissues where gluconeogenic pathway is active, the enzymes can be found in the cytosol, mitochondria, and endoplasmic reticulum (ER).
Intake of food high in fat and protein without carbohydrate induces gluconeogenesis. Key enzymes of gluconeogenesis are supposed to be present in the foetus from early in gestation and increase throughout gestation and during the neonatal period.
Conversion of pyruvate to phosphoenolpyruvate
Pyruvate carboxylase, a mitochondrial enzyme that utilizes biotin and ATP, first transforms pyruvate (made from lactate, alanine, and other amino acids) into oxaloacetate.
The inner mitochondrial membrane is impermeable to oxaloacetate. Since it can penetrate the mitochondrial membrane and be converted back to oxaloacetate in the cytosol, it is transformed to malate or aspartate.
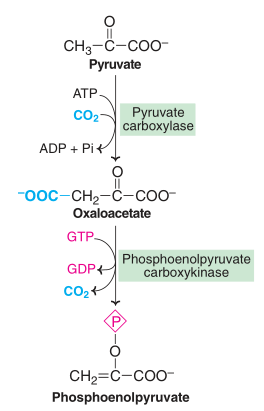
Fig: Conversion of pyruvate to phosphoenolpyruvate
Phosphoenolpyruvate carboxykinase decarboxylates oxaloacetate to produce phosphoenolpyruvate. GTP is needed for this reaction.
By switching the glycolytic processes around, phosphoenolpyruvate is transformed into fructose 1,6-bisphosphate.
Eventually, phosphoenolpyruvate is converted to fructose 1,6-bisphosphate the enzyme of glycolytic reactions.
Conversion of fructose 1,6-bisphosphate to fructose-6-phosphate
Fructose-1,6-bisphosphatase catalyzes the conversion of fructose-1,6-bisphosphate to fructose-6-phosphate, which releases inorganic phosphate.
By using the same isomerase as in glycolysis, fructose-6-phosphate is transformed into glucose-6-phosphate.
Conversion of glucose-6-phosphate to glucose
In the presence of the enzyme glucose 6-phosphatase, inorganic phosphate is released from glucose-6-phosphate, resulting in the production of free glucose that enters the bloodstream.
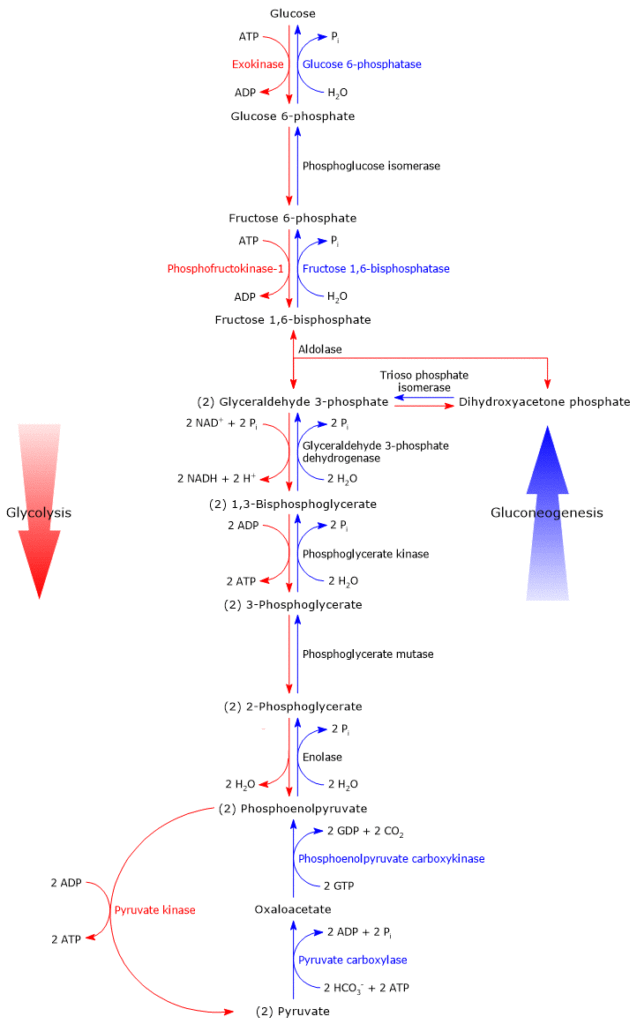
Fig: Steps involved in glycolysis and gluconeogenesis
Regulations:
Hormonal regulation
The most significant hormones influencing hepatic gluconeogenesis are insulin and glucagon. On blood glucose levels, they showed opposing effects. Under fasting or feeding, the two hormones’ blood levels will change, which will then have an impact on how gluconeogenetic genes are expressed.
Metabolic regulation
Energy conservation is crucial for organisms; thus, they have developed mechanisms to control the metabolic processes that use and release the most energy. Gluconeogenesis is inhibited when there is an oversupply of energy available. Gluconeogenesis is triggered when energy is required.
- The conversion of pyruvate to phosphoenol pyruvate is regulated by acetyl-CoA. When acetyl-CoA concentrations are high, organisms use pyruvate carboxylase to divert pyruvate from the TCA cycle because acetyl-CoA is a crucial metabolite in the TCA cycle that generates a lot of energy. If the organism doesn’t need more energy, it is always signalled such compounds toward storage or other necessary functions.
- The molecules AMP and fructose-2,6-bP negatively control and inhibit the conversion of fructose-1,6-bisphosphate to fructose-6-phosphate with the use of fructose-1,6 phosphatase. The organism enhances gluconeogenesis and decreases glycolysis when the energy levels produced are greater than what is required, or when there is a high ATP to AMP ratio. Conversely, an organism increases glycolysis and decreases gluconeogenesis when energy levels are lower than required, i.e., when the ATP to AMP ratio is low.
- Substrate level regulation governs the utilization of glucose-6-phosphatase in the conversion of glucose-6-P to glucose. The metabolite Glucose-6-P regulate this process. Glucose-6-phosphatase increases activity and more glucose is generated when levels of glucose-6-P is high and vice versa.
Significances:
- It is crucial for the control of acid-base equilibrium, amino acid metabolism, and the production of structural elements derived from carbohydrates.
- During a fast, glucagon stimulates the liver to release glucose from glycogen and produce glucose from oxaloacetate and glycerol (gluconeogenesis).
- Under anaerobic conditions, glucose is the only substance that can fuel skeletal muscle.
- Glucose serves as a constant source of energy for the brain, central nervous system, erythrocytes, testes, and kidney medulla.
- Out of the approximately 160 g of glucose needed by the total body, the human brain requires roughly 120 g daily. As a result, glucose plays a crucial role in metabolism and the body depends on a steady supply of it for a number of processes.
Gluconeogenesis Defects:
- Several inborn errors of gluconeogenesis cause hypoglycemia.
- Fructose-1,6-bisphosphatase deficiency is an autosomal recessive disorder characterized by hyperventilation associated with severe ketoacidosis, hypoglycemia, seizures, and lethargy, sometimes leading to coma.
- Lactic acidosis is caused by a deficiency in gluconeogenesis.
- The deficiency Glucose-6-phosphatase and Fructose-1,6-bisphosphatase are usually spare the brain, unless it is damaged by recurrent hypoglycemia.
- The deficiencies of Phosphoenol pyruvate carboxykinases and Pyruvate carboxylases are associated with a progressive neurodegenerative disorder which is usually fatal.
References:
- Melkonian, E.A., Asuka, E. and Schury, M.P., 2022. Physiology, gluconeogenesis. In StatPearls [Internet]. StatPearls Publishing.
- Garrett, H., Reginald and Charles Grisham. Biochemistry. Boston: Twayne Publishers, 2008.
- David, L., Nelson, D.L., Cox, M.M., Stiedemann, L., McGlynn Jr, M.E. and Fay, M.R., 2000. Lehninger principles of biochemistry.
- Satyanarayana, U., 2021. Biochemistry, 6e-E-book. Elsevier Health Sciences.
- Murray, K., Rodwell, V., Bender, D., Botham, K.M., Weil, P.A. and Kennelly, P.J., 2009. Harper’s illustrated biochemistry. 28. Citeseer, New York, United States.
- Legouis, D., Faivre, A., Cippà, P.E. and de Seigneux, S., 2020. Renal gluconeogenesis: an underestimated role of the kidney in systemic glucose metabolism. Nephrology Dialysis Transplantation.
- Berg, Jeremy M. “Glycolysis Is an Energy-Conversion Pathway in Many Organisms.” Biochemistry. 5th edition. U.S. National Library of Medicine, 01 Jan. 1970. Web. 06 Apr. 2017.
