Monomeric and oligomeric enzymes:
An enzyme that is composed of just one polypeptide chain is referred to as a monomeric enzyme, such as ribonuclease or trypsin. In contrast, when an enzyme is built from two or more polypeptide chains (subunits), it is described as an oligomeric enzyme; classic examples include lactate dehydrogenase and aspartate transcarbamoylase.
Beyond these, there exist specialized structures known as multienzyme complexes. These are large assemblies in which different catalytic sites are arranged within a single macromolecule to carry out a series of linked reactions in a stepwise manner. Importantly, the biological activity of such complexes depends on their intact, native structure—once the individual enzyme units are separated, they typically lose their functionality. Examples of these highly coordinated systems include pyruvate dehydrogenase, fatty acid synthase, and prostaglandin synthase.
Like all proteins, enzymes in general display the fundamental properties associated with proteins. However, what distinguishes a multienzyme complex is its organization: multiple enzyme activities are embedded within a single structural framework, allowing the sequential conversion of substrates to products with greater efficiency and regulation.
Inhibitors of enzyme action:
Inhibitors are physical, chemicals, and biological agents that reduce the rate of enzyme catalyzed reactions. They are usually specific and they function at low concentrations. They block the enzyme but they do not usually destroy it. Nervous system enzymes are inhibited by a variety of medications and toxins. The higher the enzyme and substrate concentration, the less effective the inhibitors are. Inhibitors are of different types:
- Competitive inhibitor
- Noncompetitive inhibitors
- Uncompetitive inhibitors
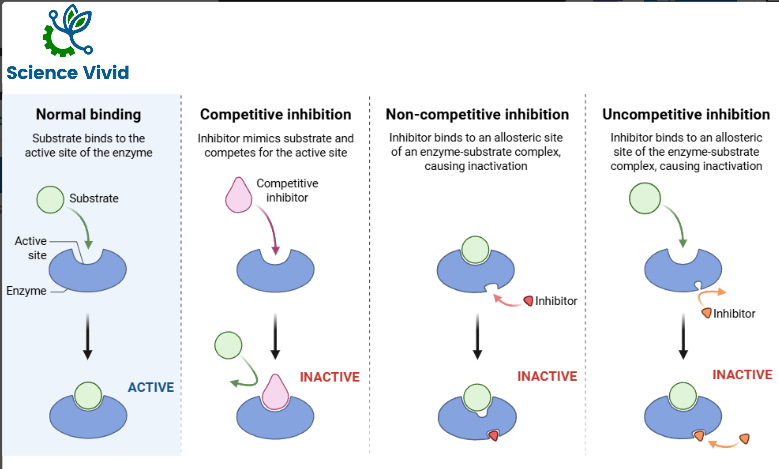
Fig: Competitive and non-competitive inhibition
Enzyme inhibition:
Irreversible inhibitors
An irreversible inhibitor will bind to an enzyme so that no other enzyme-substrate complexes can
form. It will bind to the enzyme using a covalent bond at the active site which therefore makes the enzyme denatured.
They combine with the functional groups of the amino acids in the active site, irreversibly.
Examples:
Aspirin irreversibly inhibits cyclooxygenase (COX) enzymes, which are crucial for producing prostaglandins. This inhibition lasts for the lifetime of platelets, which cannot synthesize new COX enzymes, leading to a prolonged anti-inflammatory and anti-platelet effect
Reversible inhibitors
Reversible inhibitors can be washed out of the solution of enzyme by dialysis.
There are broadly three categories.
Competitive Inhibitor
Competitive inhibitors compete with the substrate molecules for the active site. These inhibitors resemble the substrate’s structure closely. The inhibitor’s action is proportional to its concentration.
When the inhibitor binds to the active site it reduces the rate of enzyme reaction since the substrates unable to bind to the active site because the inhibitor is currently occupying the active site.
Competitive inhibitor increases Km therefore more substrate is needed to achieve ½ Vmax.
Vmax however is unaffected by competitive inhibitor.
In the Krebs cycle, malonic acid serves as a textbook example of competitive inhibition by imitating succinate and occupying the binding pocket of succinic dehydrogenase, thereby suppressing its normal catalytic role.
Methotrexate, a structural analog of folate, is widely applied in chemotherapy. By attaching to dihydrofolate reductase (DHFR), it obstructs folate metabolism, curtails nucleotide biosynthesis, and ultimately hinders cellular replication.
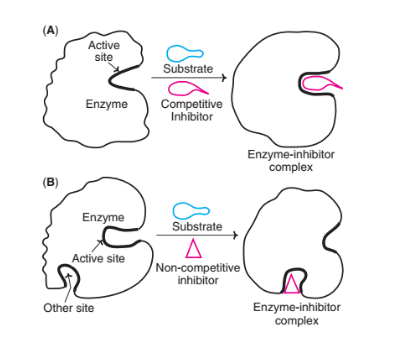
Fig: Competitive and non-competitive inhibitor
Non-competitive inhibitor
Non-competitive inhibitors bind to free enzyme or ES complex; it doesn’t bind to the active site. Therefore, the shape of the inhibitor is different from the substrate. This type of inhibitor reduces the ability of the enzyme to convert substrate into product. Inhibitor destroys the catalytic activity of the enzyme as a result of a conformational change affecting the catalytic site but does not affect substrate binding. Vmax is reduced and Km remains the same since the substrate can still bind to the active site as well as before the inhibitor is present.
Example:
Cyanide competes with oxygen for binding to cytochrome c oxidase, effectively shutting down the electron transport chain — the final step of cellular respiration. As a result, ATP synthesis grinds to a halt. Organs with high energy demand, especially the brain and heart, are the most severely damaged.
Heavy metals such as copper, silver, and arsenate act as non-competitive inhibitors of enzymes. Rather than competing with the substrate for the active site, they attach to other regions of the enzyme molecule, most commonly to sulfhydryl (-SH) groups on cysteine residues. This interaction distorts the enzyme’s natural shape, leading to changes in the active site so that it can no longer function effectively. Because of this structural alteration, the enzyme loses its catalytic ability even if plenty of substrate is available. The key effect is a reduction in the maximum velocity of the reaction (Vmax), while the substrate’s binding affinity (Km) remains unchanged. This explains why heavy metals can be toxic—they disrupt essential enzyme-controlled processes in cells.
Uncompetitive inhibitor
Uncompetitive competition occurs when the inhibitor binds only to the ES complex. They do not bind to free enzymes. Substrate binding could cause a conformational change to take place in the enzyme and reveal an inhibitor binding site or the inhibitor could bind directly to the enzyme bound substrate. So inhibition cannot be overcome by increasing the substrate concentration. It reduces both Vmax and Km in the same amount. The slope of the graph will remain constant because both the Km and Vmax will be changing proportionally to each other and so the line will simply be observed to shift up and to the left.
Example: Brefeldin A is a lactone compound with antiviral properties. It works through uncompetitive inhibition, where it traps an intermediate form of the ADP-ribosylation factor (ARF). By doing so, it interferes with normal membrane-related activities in the cell, disrupting essential processes.
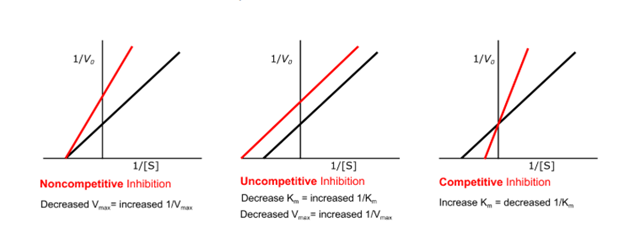
Fig: Enzyme kinetics in the presence of the inhibitor (red line) versus without the inhibitor present (black line)
