Introduction to Zika Virus and Vector-Borne Diseases:
Emergence and re-emergence of Vector Borne Diseases (VBD) have posed a huge threat to public health worldwide. Flaviviruses like Dengue Virus (DENV), Yellow Fever Virus (YFV), and Japanese Encephalitis Virus (JEV) are already circulating globally. Since the vectors, transmission cycle, favorable climate, and epidemiology of Zika virus (ZIKV) are shared by such already circulating Flaviviruses in Nepal, there is a high probability of the advent of ZIKV as a new arboviral infection in Nepal.
Transmission of Zika Virus:
Zika viruses are RNA viruses belonging to the family Flavivirus transmitted by Aedes aegypti and Aedes albopictus. Emergence of ZIKV in many parts of the world is attributed to climate change, ecological changes, and dispersion of mosquito vectors. Local changes in temperature and precipitation can continue to alter the vector dispersion and thus increase the risk of Zika virus. In India, 3 cases of ZIKV were reported in 2017. India had also declared an alert after the detection of 15 ZIKV cases in Kerala during the initial COVID-19 surge in India. India is developing more trade relationships with ZIKV-affected countries, and Nepal is a transit for trade between most of the countries. Thus, the ZIKV outbreak in India can pose a serious threat to Nepal. Like it is said, “an outbreak anywhere is potentially a threat everywhere”, ZIKV has the potential to enter Nepal as a new Arboviral infection. So, to mitigate the challenges of possible ZIKV infection in Nepal, the government should consider an integrated surveillance system and strengthen the diagnostic approaches.
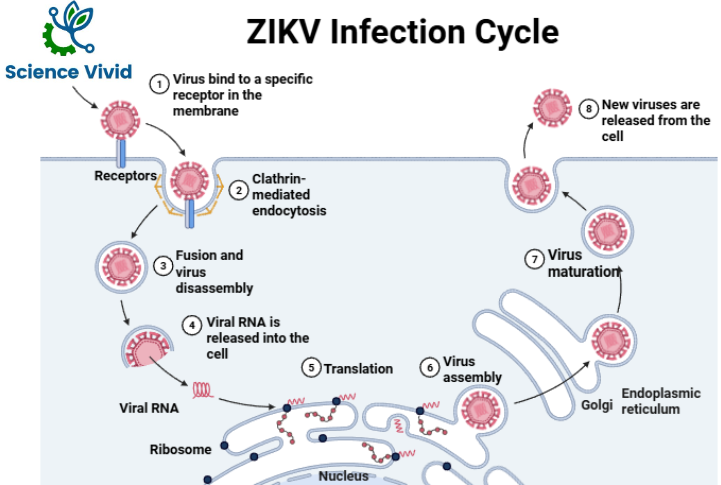
Diagnosis of Zika Virus:
Diagnosis, like all other diseases, begins with clinical diagnosis that paves the path for laboratory diagnosis. In case of ZIKV, diagnosis based on clinical presentation is not reliable because ZIKV infection shares similar clinical presentations with DENV and CHIKV infections, which are already circulating. So, laboratory diagnosis is pivotal for confirmatory diagnosis of ZIKV.
Currently, identification of ZIKV infection is achieved by
- Testing serum for presence of viral RNA using Reverse Transcriptase Polymerase Chain Reaction (RT-PCR),
- Testing serum for presence of NS1 protein, and
- Serology by Enzyme-linked Immunosorbent Assay (ELISA). For this sample can be serum, whole blood (EDTA), Cerebrospinal Fluid (CSF), urine, saliva, and amniotic fluid.
Specimen Collection and Handling:
Samples should be immediately transported to the laboratory in viral transport medium, adopting all measures for handling potentially infectious biological specimens, and the specimen workup should be performed in Bio Biosafety Level-2 (BSL-2) laboratory. Generally, during the early stages of the disease, antigen detection and RNA detection are preferred. Isolating viruses is mostly done for research purposes and is not diagnostic. Since viral antigen is detected circulating in the patient’s blood, it is helpful to detect it early in the course of the illness.
Laboratory Diagnosis Methods:
Antigen Detection Methods
Several commercial ELISA kits and Immunochromatographic strips can be used to detect ZIKV antigen marker. One study showed antibody pairs detected and distinguished NS1 proteins of DENV serotypes as well as ZIKV Non-structural 1 (NS1) without detectable cross reactions. In another study, detection of ZIKV NS3 protein antigen was done in blood monocytes, and low cross reactivity was found with DENV and YFV, indicating an anomaly in the use of antigen detection in ZIKV diagnosis unless antigen detection diagnosis is refined and strengthened.
Viral RNA Detection
Routine diagnosis by detection of viral RNA stands as the most useful method, as viral RNA can be detected during the early stage of the disease, which aids in timely patient management and avoidance of any non-conformities cost.
RNA detection can be done by Reverse transcriptase PCR targeting the nonstructural protein 5′ genomic region. It is suggested to collect serum within 5 days after symptom onset. Urine may be the specimen of choice to widen the window for ZIKV detection. Centers for Disease Control 2017 has described Trioplex Taqman RT-PCR for detection and differentiation of RNA from DENV and ZIKV in serum, whole blood (EDTA), CSF, and ZIKV RNA detection in urine and amniotic fluid.
Although RT-PCR is currently regarded as the gold standard and the FDA has approved emergency use, the CDC developed the IgM Antibody Capture ELISA (MAC), and ZIKV IgG ELISA has low specificity and cross-reactions in patients who have previously been exposed to DENV infection. IgM ELISA has high sensitivity but low specificity.
Serological Diagnosis
Serology is done after 5 days of symptom onset for ZIKV-specific IgM by ELISA or Immuno Fluorescence Assay. IgM antibody is considered a marker of recent infection. Infection detected in the early convalescent phase of infection and presence of high IgG titre or a fourfold rise in antibody titre in paired sera from the clinically suspected subject is indicative of a confirmed diagnosis. Hemagglutination Inhibition, commercial kits of ELISA and Immunochromatographic Kit using rapid strips can be used to detect IgM and IgG. However, since similar Flaviviruses are circulating, such serological reliance is complicated as antibodies against ZIKV can be reactive to those Flaviviruses to some level or even more in IgM capture ELISA and HI. In such a situation, for serology, the Plaque Reduction Neutralization Test (PRNT) could be used to differentiate antibodies of these closely related viruses, as it also gives a mathematical endpoint of antibody titer. Also, if the patient was previously exposed to heterologous Flaviviruses, interpretation could be confounded, which is a problem as other similar Flaviviruses are co-circulating.
Challenges in Clinical Diagnosis:
In a nutshell, there is a need for differential diagnosis to reliably diagnose and manage patients in the early stage to prevent mortality and non-conforming costs, which is especially true in the case of Arboviral infections with similar clinical presentations with similar vectors. Serology is seen to compromise test results with cross-reactivity. Currently, there is also a lack of sensitive antigen detection protocols for ZIKV, which necessitates the establishment of procedures to detect ZIKV NS proteins during active infection. Reliable Antigen detection tests, so developed, can be fruitful because of their low cost and rapidity.
References:
- Rijal, KR., Adhikari, B., Ghimire, B., Dhungel, B., Pyakurel, U.R., Shah, P., Bastola, A., Lekhak, B., Banjara, MR., Pandey, BD., Parker, DM. and Ghimire, P. (2021). Epidemiology of dengue virus infections in Nepal, 2006–2019. Infect Dis Poverty 10:52
- Baral, S., Uprety, S. and Lamichhane, B. (2016). ZIKA VIRUS. Health Research and Social Development Forum (HERD)
- Dhimal, M., Dahal, S., Dhimal, ML., Mishra, SR., Karki, KB., Aryal, KK., Haque, U., Kabir, M., Guin, P., Butt, AM., Harapan, H., Liu, QY., Chu, C., Montag, D., Groneberg, D., Pandey, BD., Kuch, U. and Muller, R. (2018). Threats of Zika virus transmission for Asia and its Hindu-Kush Himalayan region. Infectious Diseases of Poverty 7:40
