Introduction:
- It is the method of post-translational modification (PTM) that occur most frequently in proteins. About 50% of all proteins are glycosylated in eukaryote cells that plays a major role in protein targeting.
- It is a process where carbohydrates (oligosaccharides) are covalently attached to polypeptides, lipids, polynucleotides that is usually catalyzed in presence of glycosyltransferase enzymes, that are predominately on the outer face of the plasma membrane, in the extracellular matrix, and in the blood. Moreover, they are located in certain organelles within cells, including Golgi complexes, secretory granules, and lysosomes.
- Glycosylation of protein inner core residues occurs in the Endoplasmic reticulum (ER), whereas glycosylation of protein outer core residues may occur within either the ER or Golgi apparatus.
- Congenital glycosylation abnormalities have provided valuable insights into the fundamental mechanisms underlying the correlations of certain glycoconjugates with disease characteristics.
Types:
O- Glycosylation
Occurs post- translationally at certain serine and threonine residue takes place in Golgi apparatus.
O-linked oligosaccharides have a glycosidic bond to the oxygen atom of the hydroxyl group of amino acid including serine (Ser) or threonine (Thr) residues and N-Acetyl galactosamine (GalNAc) as the sugar at the reducing end of the oligosaccharide. GalNAc is an amino sugar derivative of galactose.
It occurs in both prokaryotes and eukaryotes.
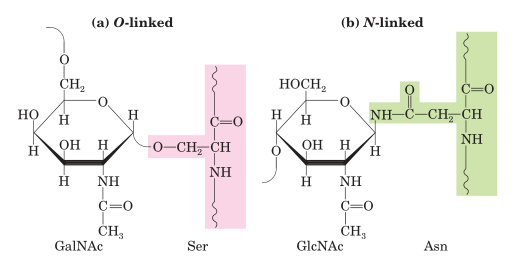
Fig: Oligosaccharide linkages in glycoproteins
N -Glycosylation
N-linked oligosaccharides have an N-glycosyl bond to the amide nitrogen of an aspargine (Asn) residue which is the most widely spread form of glycosylation occur in several secreted and transmembrane proteins.
Takes place at the interface of ER (endoplasmic reticulum) and in the Golgi apparatus mainly in eukaryotic organisms and in archaea.
C- Glycosylation
Attachment of glycans to the C2 atoms of tryptophan residues. The term “glycan” refers to molecules that include glycosidic linkages, such as sugar (monosaccharides, oligosaccharides, polysaccharides, or carbohydrates).
Biochemical Importance:
- It plays a critical role in determining protein structure, protein folding, function and stability.
- It is critical for physiological and pathological cellular functions maintaining cellular homeostasis.
- Glycosylation is required for a variety of biological functions, including cell adhesion to the extracellular matrix and protein-ligand interactions within the cell.
