Introduction:
It is a technique which us the spring property of an electron which are in resonance. It is based on the paramagnetic behavior of an electron which are in resonance. All molecules have an atom and every atom have a nucleus. Every nucleus contains proton and neutron. Neutron is neutral and proton is positively charged in nature. So, the overall charged of the nucleus is due to neutron. So, Proton is positive the nucleus is also positively charged body.
Around the nucleus, negatively charged electron are present. Electron has the property to rotate around the nucleus and electron also rotate around its own axis. So, there are two types of rotation performed by an electron. So, when the charged body rotate, it behaves like a magnet and generate magnetic field.
So, we can observe that the electron is negatively charged and it is rotating around its own axis. So, electron also behave like a magnetic and generate magnetic field. So, the magnetic property of the rotating electron helps in working of ESR/EPR.
Generally, when the electron is rotating around its own axis, it behaves like a magnet. So, there are two types of magnetic property performed by rotating electron.
Diamagnetic and paramagnetic
Generally, molecules contain pair of electrons. In the paired electron, one electron is rotating in clockwise direction and other in anticlockwise direction. If the rotation is in a clockwise direction, it magnetic field is indicated by Upward ↑ and if anticlockwise, it is denoted by↓
So, the magnetic field generated by the two electrons will have oppositive rotation having antiparallel magnetic field. Due to the antiparallel magnetic field, the magnetic field generated by an electron will cancel each other magnetic field. So, there is a self-cancellation of magnetic field by two electrons which have opposite magnetic field.
To explain the rotation of an electron around its own axis we use a special term known as Electron spin quantum number which is represented by MS= +1/2, MS= -1/2. If the electron is rotating in a clockwise direction, it has MS= +1/2, and if in anti-clockwise it is denoted and as MS= -1/2. So, due to the opposite the opposite rotation self-cancellation occurs and such kind of behaviors of an electron is known as Diamagnetic behaviors of an electron. Paramagnetic behaviors are generally shown by an electron which have odd number of electron (unpaired electron). There is no self-cancellation on rotating electron. Paramagnetic behavior is shown by metal like Fe, cobalt, Nickel, free radicals also electron in a triplet state. The paramagnetic behaviors of these elements are known as ferromagnetism. In ESR, only paramagnetic electron is used because in an applied magnetic field only paramagnetic electron are attracted. So, ESR is also known as EPR (Electron Paramagnetic Resonance).
Principle:
Applied magnetic field is supplied that generate a magnetic field of 50-500 Tesla mT, 3400 Gauss. A Microwave of 3cm and 9GhZ will be introduced and a molecule with unpaired electron.
After alignment, the direction of an applied magnetic field, direction of magnetic field generated by an electron spin in the same direction which is known as Alpha State/ Parallel State/ Lower energy state.
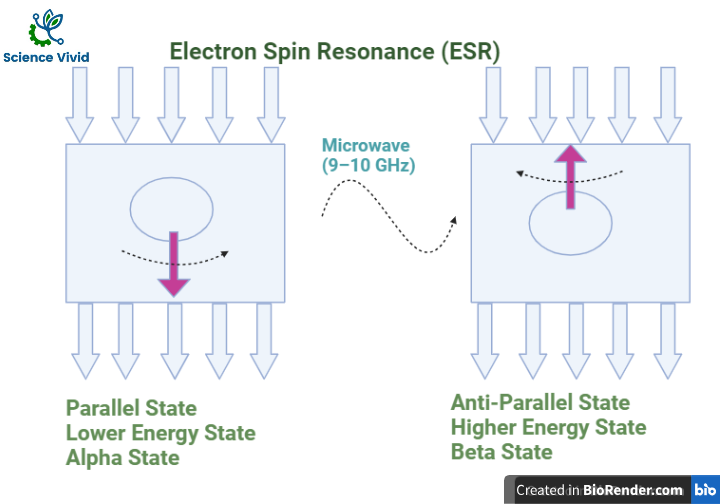
Fig: Electron Spin Resonance Spectroscopy
In the next step, same energy is applied provide to an electron which are present in the alpha state. So, when microwave radiation incident in the electron, the electron is now able to oppose direction of magnetic field. Now, it just will change its spins and shift its position. Such condition is known as Beta State/ Antiparallel State/ Higher energy state.
The change of direction of the spin of the electron is known and the resonance and the change are shown by an electron and the technique used to study the spin property of electron which causes resonance is known as ESR.
If the supply of the energy from microwave is stopped, the electron which are present in a beta state will again come back to the alpha state.
Instrumentation:
A typical ESR spectrometer contains the following main components:
- Source (Klystron, isolator, wavemeter, and attenuator)
- Sample cavity
- Magnetic system
- Crystal detectors
- Auto amplifier and phase-sensitive detector

Fig: Electron Spin Resonance Spectroscopy
Klystrons
This tube acts as the source of radiation which is stabilized against temperature fluctuation by immersion in an oil bath or by forced air cooling. The applied voltage (300 milli watts) to klystron determined the frequency of the monochromatic radiation.
Oscillators is generally used in order to minimizes vibrations and stabilize in the frequency of microwaves generated by Klystron.
Sample cavities
It is the cavity the contain the sample. Generally, solid form of sample is used. In cases, if liquid nitrogen is used. Cryopreservation is generally used to convert liquid sample into crystal form. DPPH (2,2-Diphenyl-1-picrylhydrazyl) is generally used as reference sample. Rectangular cavity and cylindrical cavity have widely been used. The sample is generally placed where the intensity of magnetic field is greatest because the applied magnetic field need to interact with the sample to cause resonance.
Magnet system
The sample cavity is placed between the two pieces of an electromagnet and the magnetic field need to be stable and uniform. Moreover, the stability of field is achieved by energizing the magnet with a highly regulated power supply.
Crystal detectors
Silicon crystal detectors that convert the radiation in direct current (D.C) has widely been used as a detector of microwave radiation.
Autoampilfier and phase-sensitive detector
After being detected by the crystal detector, the signal is amplified by a narrow-band amplifier, which produces a lot of noise. Eventually, the phase-sensitive detector will help to minimize noise.
Applications:
- Detection of types and number of free radicals in the biological samples. Free radicals are hhighly reactive species cause damage to biomolecules such as DNA, proteins, and lipids.
- It can be used to study the electronic properties of metalloproteins and metalloenzymes.
- Detection of Transition Metal Ions such as ions such as iron, copper, and manganese.
- Study of radicals involved in Photosynthesis:
- Study of role of oxygen radicals in development of diseases such as cancer, atherosclerosis, and neurodegenerative diseases.
