Aim:
To separate green leaf pigments (Spinach leaves) by Thin Layer chromatography (TLC) method.
Introduction:
It is a type of planar chromatography, in which the stationary phase is a thin layer of adsorbent particles attached to a solid plate. A small amount of sample/analyte is applied (spotted) near the bottom of the plate (an imaginary line) using capillary tube. Eventually, the plate is placed in the solvent, which is the mobile phase of the chromatography system. This solvent in the chromatography chamber is drawn up by capillary action and under the principle of partition coefficients.
Separation occurs as each component, being different in chemical and physical properties, interacts and distribute within the stationary and mobile phases to a different degree, developing the individual bands on the plate. Likewise, after the completion of the process, Retardation factor (Rf) value is used to characterize and compare components of various samples.
Retardation factor (Rf) = Distance travelled by analyte /Distance travelled by solvent
Requirements:
Glass plate, silica gel, distilled water, ethanol and chloroform, spinach extract, chromatographic chamber.
Procedure:
Extraction of the leaf pigments
Firstly, collect fresh plant leaves (e.g., spinach, lettuce, or any green leafy vegetable) and remove any dirt or debris and cut the leaves into small pieces using scissors. Take 70 g of spinach and gently crush it with 60 ml methanol/acetone/ethanol and 3ml of petroleum in mortar and pestle. (Mixture of 30;40 methanol: petroleum ether).
Filter the contents of mortar through cotton wool or filter paper. It is believed that the carotenoids will dissolve in the petroleum ether layer. Preserve the filtrate. Reground the residue again and again filter and separate. Wash the petroleum ether layer twice with water to remove any dissolved methanol. If you have used petroleum ether or if there are solid particles after filtration, we can use a centrifuge to separate the layers. Finally, centrifugation helps in obtaining a cleaner extract which can be used as a leaf extract for the further separation.
Developing solvent (mobile phase)
100 mL of petrol ether, 11 mL of isopropanol and 5 drops of distilled water. Since, petroleum ether is volatile and very flammable, it presents a high fire risk. It is always recommended to avoid direct contact, ingestion and inhalation. In addition, acetone and isopropanol are highly also flammable, So, the precautions measures need to be strictly followed.
Preparation and Activation of TLC Plate
TLC Slurry preparation for soft layer coating: Silica Gel and small amount of calcium sulphate (gypsum) is mixed with water. This mixture is spread as a thick slurry on a clean glass plate. After that, plate is dried and activated by heating in an oven for thirty minutes at 110°C.
Sample Application
Using a pencil, a line is drawn approximately 1.5 cm from the bottom of the plate. Capillary tube or Pasteur pipet the leaf extract is applied as a line to the TLC plate and the applied extract is allowed to dry thoroughly.
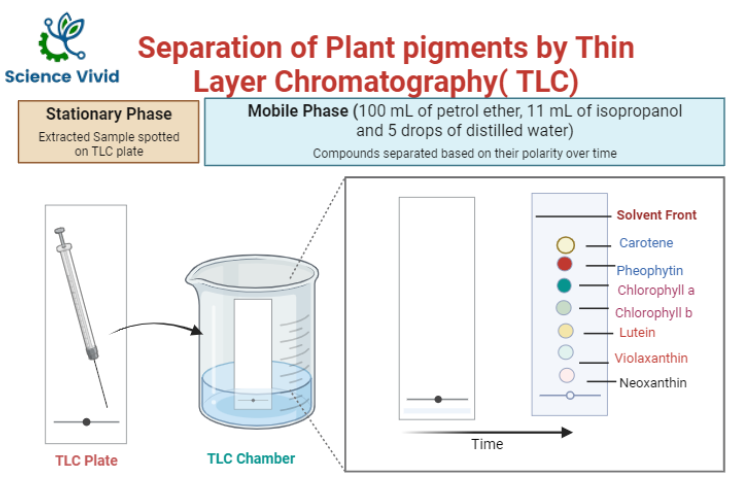
Fig: Separation of Plant Pigments by Thin layer chromatography (TLC)
Result and Interpretation
After the run of the solvent in plate under the chromatographic chamber. A solvent front is marked and retardation factor is calculated for each separated pigment. Visualization of the separated pigments can be performed using a UV lamp. Numerous pigments of plant that include chlorophyll, fluoresce under UV light, which make them visible as distinct bands. In some case of iodine vapour chamber which is prepared with iodine in ethanol has also been used for visualisation.
