Introduction:
Cell senescence can be defined as a process occurring in all members of population, after maturity, involving progressive decline in vital capacities of the organism termination in death. Senescence is also considered as death.
The science that studies biological caused of growth. At the cellular level senescence can be studied on the basis of three process.
- Decline in the final efficiency of non-dividing highly specialized cells such as neurons and muscle cells to the possible extent.
- Progressive stiffening with age of the structural proteins-such as collagens.
- Limitation imposed on cell division as revealed by the studies on fibroblast producing collagen and fibroin.
Mechanisms of cell ageing:
It can be suggested that normal cells have a finite capacity for replication, and this finite limit is rarely reached in vitro but is, of course, demonstrable in vitro. The functional loses that occur in cells prior to their loss of division capacity produce age changes.
According to L. Orgel (1963), cellular ageing results from impaired specificity of the translation step in protein synthesis. This hypothesis has been confirmed to a great extent.
Theories of senescence:
Many theories related to cell senescence depending upon the different types of nucleic acid and nucleoprotein structures are describes.
- Change in nucleic acid quantity: Loss of DNA or RNA per cell organ could explain decreasing functional efficiency with increasing age. Histological findings point to progressive loss with age of certain irreparable types of cells.
- Mutation theory: The effect of mutations is to bring about the synthesis of faulty messenger RNA and thus, in turn, faulty proteins which are unable to fulfill their biological functions or can do so only imperfectly. Due to faulty proteins, impaired specificity of the enzymes is formed in the translation mechanism, according to Orgel.
There are many ways in which proteins synthesis-information content can be changed, falsifies or diminished. The observation and results of many biologists’ are mentioned below:
- In ageing cell replacement of defective molecules of metabolic DNA becomes impossible, therefore defective molecules accumulate; as a result of many faulty DNA molecules in the cell, functional impairment takes place.
- Ageing is attributed to loss of repetitive information.
- The number of methyl group (5’ methyl cytosine) in DNA decreases with age, thus DNA information content gets modified and affects protein synthesis.
- Modification in protection regulatory mechanisms; According to Orgel, ageing of cells may ensure as a result of cumulation of transcription and translation errors in protein synthesis. Errors leading to reduced specificity of an information handling enzyme leas to an increasing error frequency. Such process is cumulative. It is a question whether cellular ageing may be due to failure or switchover regulatory process. Could there be an ageing programme? As an answer to this DNA pool in old age has been observed, and a hypothesis was formulated that each species has species specific programme that serves to maintain and prolong useful life. It is also proven that there is a shift from, arginine-rich to a lysine rich histone composition in the liver of old animals.
Free radical theory of ageing:
The aging process can be separated into two types of cumulative degradative alterations.
- Wide damage produced by a variety of means, such as autoimmune reactions, ionizing radiations and pollution.
- Alternations in the biological clocks-determine the maximum life span of an individual cell.
The high reactivity of free radicals is due to the presence of a free electron. Due to the magnetic moment, associated with a free electron, free radicals can be detected at a low concentration using the technique of electrons spins resonance (ESR) spectroscopy.
Free radical reactions rate, involving molecular oxygen is accelerated by catalysts such as copper, iron and manganese; and inhibits by antioxidants such as vitamins E, butylated hydroxytoluene, and 2- mercapto ethylamine (2-MEA) which are capable of removing intermediate free radicals. These compounds are expected to minimize deleterious effect of free radicals. Vitamin E is natural antioxidants which has a modest beneficial effect on life span as it decreases the rate of free radical formation.
Senescence and Immunological surveillance:
Senescence process is basically related to the fate of the less differentiated mesenchymal cells responsible for the functions of defense and repair with lymphocytes and fibroblasts. These cells, like all somatic cells, are prone to mutations. Though genetic mutations in the somatic cell may modify any cellular activity, they cause two major changes.
- Against the changed cells, altered antigens generate immune responses and
- Mutations within lymphocytes induces a change in tolerance to sled components.
Somatic mutation hypothesis:
Every living species has certain life span, beyond which genetically controlled programming becomes less effective until death. Most vulnerable material to age related damage is the genetic material, i.e., DNA. Changes in the DNA base sequence alter the template and, as a result, affect the regulatory and metabolic capacities of the cell. Cell genome damage may occur in several ways, such as radiations or multiplying errors caused by unmatched nucleotides. Somatic mutation continuation involves error in the structure of enzymes connected with protein synthesis, causing an irreparable damage that will lead to failure of ell mitosis.
Thymus Function:
Thymus is a very important “Biological clock” which allows phenotypic expression of genetically decided age. It is a known fact that serum antibodies are produced only by B-lymphocytes and most natural antigens can stimulate antibody production exclusively by the cooperation of T and B immunocytes. T-immunocytes (thymus determined lymphocytes) without B-cell co-operation will lead to delayed hyper sensitivity, for homograft rejection and for medical examination important segment of immunity against viral and mycobacterial infections. Immunological inadequacies of old age involve weakness of T-cell rather B-cell function. So, sue to losing flesh of thymus in middle age, T-cell loses to incorporate effective immunocyte clones against antigens which were previously not encountered.
Immune surveillance:
Somatic mutations are neither continuous nor heritable changes in a cell line. The development of new change, benign or malignant, must be considered as somatic mutation. Such modifications are sometimes involved with some degree of antigenicity.
Common types of cancer can be considered as old age disease in which any directly influencing carcinogenic agents are very common to have influence on incidence. Senescence may be assumed to act in two ways:
- By allowing time for may mutation to occur.
- Because of the increasing weakening of immunological surveillance with age.
Senescence of connective tissue:
The connective tissues of the body show significant age-related changes. All the forms of connective tissues, Viz., dermis, tendons, cornea, vascular walls, cartilages, bones, etc., are derived from embryonal mesenchymal. All these connective tissues possess large multicellular spaces filled with collagen, elastin, proteoglycans and structural glycoproteins, collectively considered as intercellular matrix macromolecules (IMM).
In the beginning of embryonic life proteoglycans and structural glycoproteins participate actively in differentiation process and later this role is taken over by collagen and elastin. In adult life, collagen functions whenever necessary, whereas elastin is suppressed. At an advanced stage, collagen synthesis reduces rapidly.
Senescence of elastin Tissue:
Elastin fibers play an important role in elasticity and normal tone of skin and blood vessels. Quantity of these elastin fibers deteriorates with age in skin, blood vessels, particularly during arteriosclerosis and ageing disease. Degradation of elastin fibers start usually at a relatively young age, but at the age of 45 years, the degradation accelerates.
Ageing and termination of synthesis program:
Translation and transcription regulations at genetic level determine the speed of ageing process, and if this control is upset, the cells may synthesize wring molecules or stop synthesizing at all. This process may be either due to exhaustion of genetic program or to the collection of errors.
Furthermore, another mechanism that is dependent on the cellular micro and macro environment, nutrition, physical activity, and a balanced diet, among other things, may contribute to aging senescence. Avoidance of regular exercise damages healthy functioning of muscle tissues.
Apoptosis:
Multicellular organism, homeostasis is maintained through a balance between cell multiplication and cell senescence (Cell death). Physiologically cell death occurs mainly through a process apoptosis. Recent evidences suggests that alternations in cell survival contribute to pathogenesis if a variety of human diseases including cancer, viral infections, autoimmune diseases, neurodegenerative disorders and AIDS. Treatment established to alter the apoptotic threshold may cure some of these diseases (Thompson 1995).
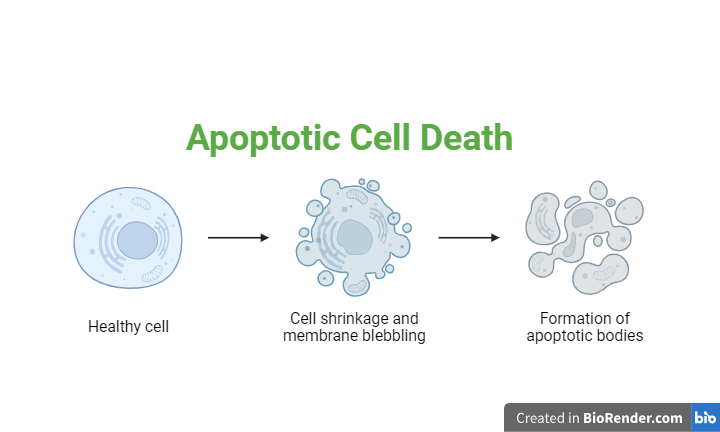
Fig: Apoptotic Cell Death
Apoptosis is a kind of programmed cell death which plays an important role during development, homeostasis, and in several diseases like cancer, AIDS and neurodegenerative disorders. It operates through the activation of a cell intrinsic suicide program.
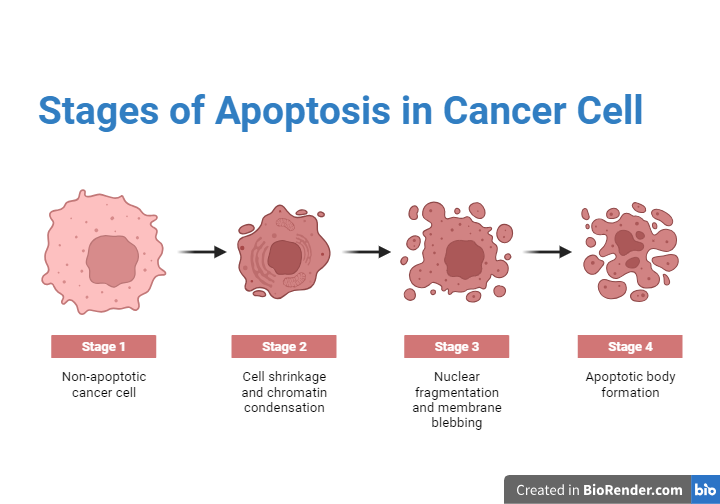
Fig: Stages of Apoptosis in Cancer Cell
