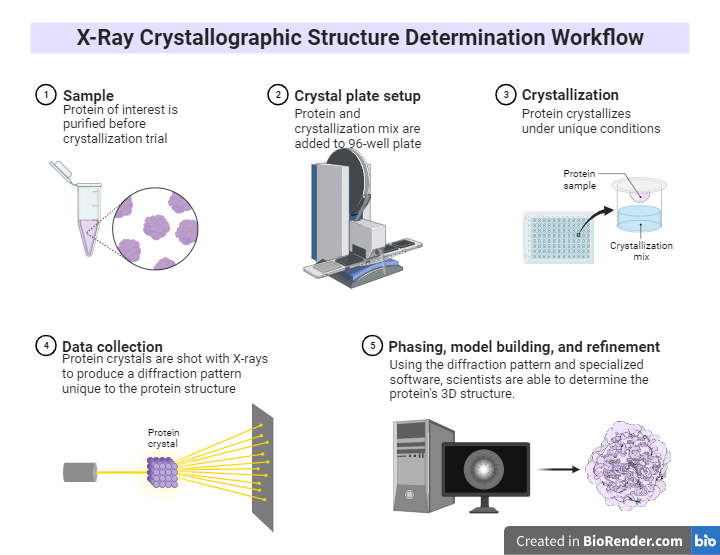
Introduction:
X-ray crystallography is a tool used for determining the atomic and molecular structure of a crystal. The underlying principle is that the crystalline atoms cause a beam of X-rays to diffract into many specific directions.
Principle:
The atoms of crystal diffract X-rays to several specific directions, and the intensity and angle of the diffracted beams generate a three-dimensional (3D) electron density image from which the mean position of atoms in a crystal, their chemical bonds, and disorder can be determined. X-ray crystallography still remain the most effective approach for determining the structure of macromolecular crystals such as proteins, DNA, medicines, and vitamins.
Instrumentation:
X-ray Source:
X-ray crystallography requires a source of X-rays. Typically, this is an X-ray tube that produces X-rays when high-energy electrons strike a metal target (usually copper or molybdenum). The choice of target material affects the characteristics of the X-rays produced. The X-rays generated by the X-ray tube are polychromatic, meaning they consist of a broad range of wavelengths. A monochromator is used to select a single wavelength of X-rays, improving the quality of the diffraction pattern. The collimator focuses and directs the X-ray beam onto the crystal. This helps in obtaining a sharp and well-defined diffraction pattern.
Sample Holder:
The crystal to be studied is mounted on a sample holder. The crystal must be of high quality and well-ordered to produce a clear diffraction pattern.
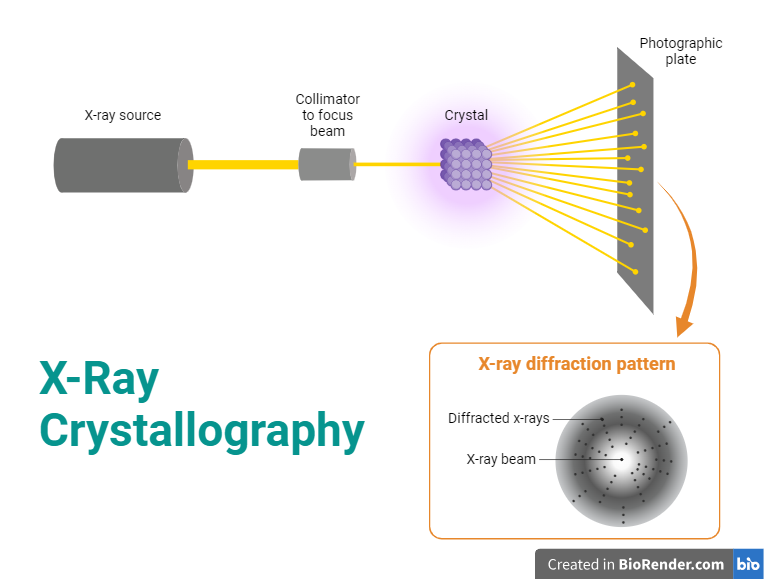
Fig: X-Ray Crystallography
The goniometer is used to position the crystal accurately in the X-ray beam, allowing for the collection of diffraction data at different angles. This is crucial for obtaining a complete set of diffraction information. The goniometer is used to position the crystal at selected orientations.Goniometer is like a high-tech sample holder; it serves to hold the crystal in the beam of X-rays, but it also rotates the sample to precise degrees.This allows the crystal to be struck by X-rays in the many different orientations needed to collect enough diffraction data for quality analysis.One of the most common types of X-ray diffraction goniometers used today is known as a kappa goniometer.
Detector:
The diffracted X-rays are captured by a detector. Modern X-ray crystallography often uses area detectors such as CCD (Charge-Coupled Device) or CMOS (Complementary Metal-Oxide-Semiconductor) detectors. These detectors allow for the simultaneous collection of a large amount of data and are more efficient than older methods that used film.
Data Collection System:
The data collection system records the intensity and position of diffracted X-rays. The goniometer moves the crystal through a series of angles, and at each angle, the intensity of diffracted X-rays is measured.
Data Processing Software:
Specialized software is used to process the collected data and convert it into an electron density map. This process involves Fourier transformation and other mathematical techniques to convert the diffraction pattern into a three-dimensional electron density map.
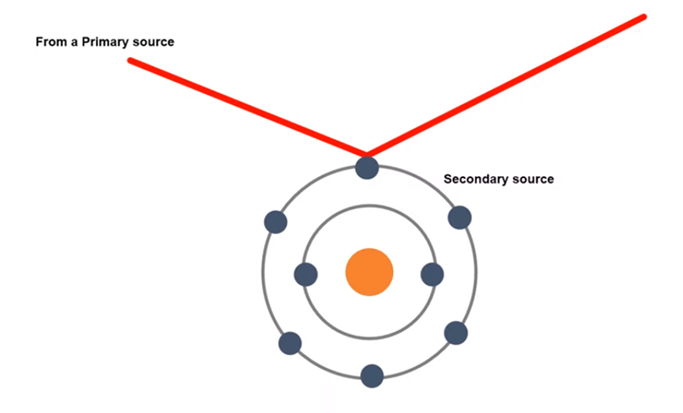
X- ray:
X ray is an electromagnetic wave whose wavelength is less than have of ~1 Å or 0.1 nm. When the wave will be incident in the atomic electron, the electron will be absorbed and vibrate accordingly. As electron is a charged particle, a charged particle will vibrate and emit the electromagnetic radiation. The electron will absorb the x ray from the primary source, vibrate and emit accordingly. But the source of emitted radiation will be electron which is known as the secondary source. This phenomenon is known as scattering of X- ray by atomic electron.
Bragg’s law:
The incident of x-ray and diffracted angle of incident is defined by Bragg which is known as Bragg’s law. As per the law, if the if the X-ray incident with the angle of Ө, then after the refraction it will be detected in the detector. This incident angle is known as Bragg’s angle and angle between the incident wave and diffracted wave is known as diffraction angle. In order to illustrate, the brag consider that the arrangement of atom crystal a is like a parallel plane. X ray beam will incident in the parallel form in the Ө angle. The interplanar spacing parallel plane is represented as “d”. The both rays will incident in the atom and reflected in the same directions and interference will be depend upon the path differences of both the wave. Path difference refers to the difference in the distances travelled by two waves from their sources to a specific point.
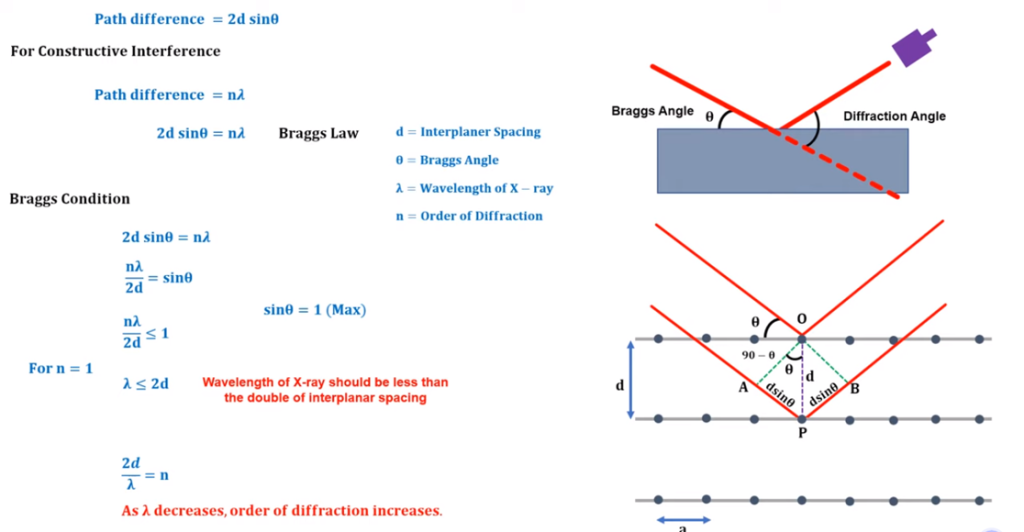
Fig: Bragg’s law
Order of diffraction
- n=1 (only one peak will be available/ visualize in the detector)
- n=2 (only two peak will be available/ visualize in the detector)
- n=3 (only three peak will be available/ visualize in the detector)
In the screen of detector, we will observe the peaks of the diffractions.
Note:
The number of peaks is directly proportional to the order of diffraction. But, the increase in the order will mixed the peaks will be very difficult to separate through analysis and interpretation. Therefore, there is a Bragg’s condition.
Workflow:
Crystals:
Solids are of two types.
- Amorphous solids-solids of with disordered arrangement of molecules, and produces all types of patters.
- Crystalline solids-solids with ordered arrangements of molecules, e.g., Quartz crystal a produces smaller crystal of the same shape.
Crystals are solids that are exact repeats of symmetric motifs that pack in exact patters and form specific shaped structures.
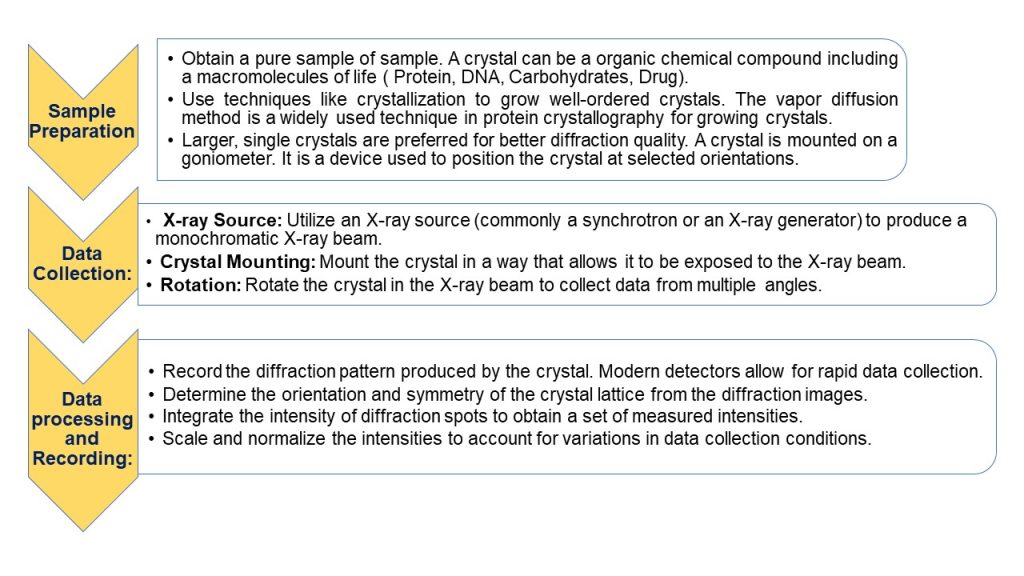
Fig: X-Ray Crystallographic Structure Determination Workflow
Applications:
Determining Molecular Structure
It is widely used to determine the three-dimensional structure of chemical compounds that includes both organic and inorganic molecules. at the atomic level.
Biological Macromolecules
The structures of large biological molecules such as proteins, nucleic acids, and large complexes like ribosomes are explained by this technique.
Drug Design
It plays a pivotal role in drug discovery by knowing the three-dimensional structure of a biological target (e.g., a protein associated with a disease) allows for the design of more effective and selective drugs.
Protein Engineering
It plays a major role in protein engineering that help to know the structure of protein. Eventually, the research can modify the structural organisation of protein as per the requisite
