Introduction:
- A chemical reaction catalyzed by an enzyme is known as an enzyme reaction. Enzymes are biological catalysts that speed up chemical reactions by decreasing the activation energy needed for the reaction to take place. Enzymes are proteins with specialized structures that allow them to attach to a substrate molecule at their active site, which is where the chemical reaction occurs.
- The substrate molecule attaches to the enzyme’s active site during an enzyme process, establishing an enzyme-substrate complex. The enzyme subsequently catalyzed a chemical process that converts the substrate into a product that is released by the enzyme. The enzyme is not consumed during the reaction and can hence catalyzed further reactions.
- Several biological functions, such as metabolism, DNA replication, and protein synthesis, rely on enzyme reactions. Enzymes can be substrate specific, catalysing just reactions involving that substrate, or they can be more generic, catalysing reactions involving numerous substrates.
- A multitude of factors can influence the rate of an enzyme-catalyzed reaction, including the concentration of the substrate and enzyme, the pH and temperature of the reaction environment, and the presence of any enzyme inhibitors or activators. Understanding the mechanics and kinetics of enzyme reactions is critical in the development of medications, diagnostic tests, and industrial operations that depend on enzymatic catalysis.
Single substrate enzyme reaction:
It is a form of enzyme-catalyzed reaction in which only one substrate molecule binds to the active site of the enzyme and undergoes a chemical change to produce a product. The enzyme works as a catalyst in this reaction, lowering the activation energy required for the substrate to undergo the chemical reaction, resulting in a quicker rate of substrate conversion to product.
The enzyme sucrase converts sucrose to glucose and fructose as an example of a single substrate enzyme reaction. At the active site of the enzyme, it binds to the sucrose substrate and cleaves it into two products, glucose and fructose, which are then released from the enzyme. The enzyme is unaltered and ready to catalyzed new reactions.
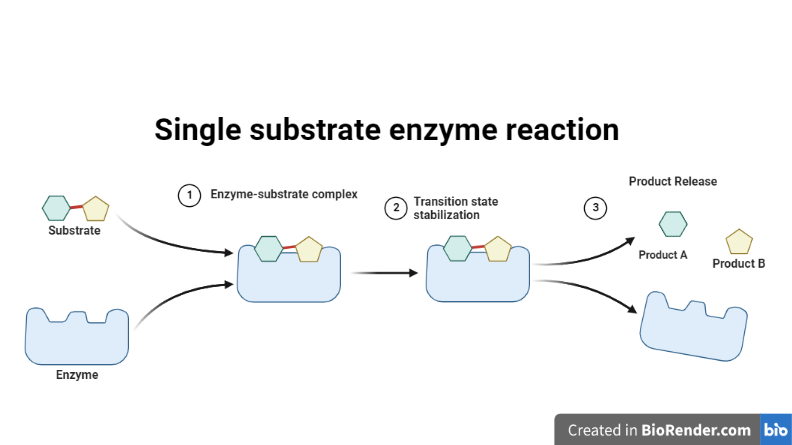
Fig: Single substrate enzyme reaction
Another example of a single substrate enzyme reaction is the conversion of lactose to glucose and galactose by the enzyme lactase. In this reaction, the enzyme binds to the lactose substrate at its active site and breaks the glycosidic bond between the glucose and galactose molecules, releasing them as separate products.
Single substrate enzyme reactions are often used in diagnostic assays to measure the presence or amount of a particular substrate or product in a sample. For example, the activity of the enzyme creatine kinase can be measured in blood samples to diagnose heart attacks, as the enzyme catalyses the conversion of creatine phosphate to creatine and ATP, which is released into the blood when heart muscle cells are damaged.
Multisubstrate enzyme reaction:
It is an enzyme-catalyzed reaction involving many substrates. Two or more substrates are attached to the enzyme simultaneously and undergo chemical change to form products in this sort of reaction.

Fig: Multisubstrate enzyme reaction
Multisubstrate enzyme reactions are classified into two main categories: sequential reactions and ping-pong reactions.
Sequential reactions
All substrates bind to the enzyme before any product is released in these reactions. Before being released as products, the substrates are changed through a sequence of intermediary reactions. These reactions might occur at random or in an ordered manner. The substrates in random reactions can bind to the enzyme in any sequence, but the substrates in ordered reactions must bind in a specified order for the reaction to occur.
There are two types of sequential multisubstrate enzyme reactions: ordered and random reactions.
Ordered sequential reactions
The substrates bind to the enzyme in a certain order in these sequential reactions, and the products are also released in a specific order. The enzyme contains two distinct binding sites for the substrates, and binding of the first substrate causes a conformational change in the enzyme, enabling binding of the second substrate easier.
For example, the enzyme alcohol dehydrogenase, which converts ethanol to acetaldehyde, follows an ordered sequential reaction mechanism. In this reaction, NAD+ (nicotinamide adenine dinucleotide) binds to the enzyme first, followed by ethanol. After the reaction occurs, the products acetaldehyde and NADH are released in that order.
Random sequential reactions
The order of substrate binding is not defined in these sequential processes, and any substrate can bind to the enzyme first. Nonetheless, the products are still released in a certain order.
For example, the enzyme creatine kinase, which catalyses the transfer of a phosphate group from ATP to creatine, follows a random sequential reaction mechanism. In this reaction, both ATP and creatine can bind to the enzyme first, and after the reaction occurs, the product phosphocreatine and ADP (adenosine diphosphate) are released in that order.
Ping-pong reactions (Double displacement reaction)
These are multisubstrate enzyme reactions in which one substrate is converted into a product before the enzyme binds to the second substrate. After the first product is released, the enzyme undergoes a conformational shift, allowing the second substrate to bind and undergo transformation. Because the first substrate is displaced by the first product before the second substrate attaches, this reaction is also known as a “double-displacement” reaction.
Ping-pong reactions involve a single active site on the enzyme that can bind to both substrates sequentially. The reaction proceeds in two steps: the first substrate is modified and released from the enzyme, and then the second substrate binds and undergoes further modification to produce the second product.
An example of a ping-pong reaction is the conversion of glutamine to glutamate by the enzyme glutaminase. In this reaction, the enzyme binds to glutamine and removes an amide group, producing glutamate and ammonia. The enzyme then releases the glutamate product before undergoing a conformational change that allows it to bind to another molecule of glutamine.
Another example of a ping-pong reaction is the conversion of fructose-6-phosphate to glyceraldehyde-3-phosphate and dihydroxyacetone phosphate by the enzyme aldolase. In this reaction, the enzyme first binds to fructose-6-phosphate and cleaves it into two products, which are then released. The enzyme then undergoes a conformational change that allows it to bind to a molecule of glyceraldehyde-3-phosphate, which undergoes further modification to produce dihydroxyacetone phosphate.
References:
- Akerman SE, Müller S (August 2003). “2-Cys peroxiredoxin PfTrx-Px1 is involved in the antioxidant defence of Plasmodium falciparum”. Molecular and Biochemical Parasitology. 130 (2): 75–81
- Kraut J (1977). “Serine proteases: structure and mechanism of catalysis”. Annual Review of Biochemistry. 46: 331–358.
- Dhatt, S., Bhattacharyya, K. Single-substrate enzyme kinetics: the quasi-steady-state approximation and beyond. J Math Chem 51, 1467–1477 (2013).
