Introduction:
A solution is a homogeneous mixture of two or more substances. In a solution, the solute (the substance being dissolved) is evenly distributed throughout the solvent (the substance doing the dissolving). Solutions can be liquid, gaseous or solid. A solution can be in equilibrium where both the dissolved and undissolved components are present in a state of balance.
Examples of solutions include salt water, sugar in water, and air (a mixture of gases). Solutions can be created through the process of dissolution, where the solute is added to the solvent and the mixture is stirred until the solute is fully dissolved.
Components:
A solution is made up of two main components: the solute and the solvent. It’s important to note that the components of a solution are not chemically bonded or changed in any way. They are simply mixed together and can be separated again through physical means, such as evaporation or filtration.
- The solute is the substance that is being dissolved in the solution. It is present in smaller amounts compared to the solvent.
- The solvent is the substance that is doing the dissolving and is present in larger amounts compared to the solute. It is the medium in which the solute is dissolved.
- In some cases, there can be more than one solute and solvent in a solution. For example, in a ternary solution, there are three components: a solute and two solvents.
- Additionally, when a solution is in equilibrium, the concentration of the dissolved and undissolved components is in balance.
Types:
Ideal solution: A solution in which the behavior of the solution can be predicted using the ideal solution theory. In this type of solution, the interactions between the solute and solvent particles are identical to those in the pure solvent and solute.
Non-ideal solution: A solution in which the behavior of the solution cannot be predicted using the ideal solution theory. In this type of solution, the interactions between the solute and solvent particles are different from those in the pure solvent and solute.
Electrolyte solution: A solution that contains ions and can conduct electricity. Electrolyte solutions are formed when an ionic compound dissolves in water, and can be either strong or weak.
Colloidal solution: A solution that contains particles that are intermediate in size between those of a true solution and a suspension. Colloidal solutions appear cloudy or opaque, and the particles do not settle out over time.
Supersaturated solution: A solution that contains more solute than it can hold at a given temperature, resulting in the solute being in a metastable state. Supersaturated solutions can be made by rapidly cooling a hot solution or by adding more solute to a cooled solution.
Hyper saturated solution: A solution that contains more solute than it can hold at a given temperature and pressure, resulting in the solute being in a stable state. Hyper saturated solutions can be made by adding more solute to a cooled solution and raising the pressure.
Stock Solution: A stock solution is a concentrated solution that is prepared in a large amount and can be used to prepare other solutions. It is a convenient way to handle and store large amounts of a particular chemical or reagent.
A stock solution contains a known and high concentration of a solute and is prepared using a suitable solvent. The stock solution can then be used to prepare other solutions of known concentrations, called working solutions. The working solutions are typically made by diluting the stock solution with a solvent.
For example, a stock solution of HCL can be prepared by dissolving a known amount of HCL in water, and this stock solution can be used to prepare a variety of working solutions of different concentrations, such as 0.1 M, 0.01 M and 0.001 M, by diluting it with water in different proportions.
Unsaturated solution: A solution that contains less solute than it can hold at a given temperature and pressure, resulting in the solute being in a stable state. Unsaturated solutions can be made by adding more solvent to a cooled solution and lowering the pressure.
Working solution: A working solution is a solution that is prepared using a stock solution. It is a solution of known concentration that is used in various laboratory experiments or procedures. The concentration of a working solution is typically lower than that of the stock solution it is prepared from.
Working solutions are typically made by diluting a stock solution with a solvent. For example, a stock solution of HCl can be used to prepare a variety of working solutions of different concentrations such as 0.1M, 0.01M, and 0.001M by diluting it with water in different proportions.
Working solutions are typically used for specific purposes, such as titrations, chemical reactions, or experiments. They are usually prepared immediately before use and are not stored for long periods of time.
Units:
The concentration of a solution is usually expressed in terms of the amount of solute that is present per unit volume or unit weight of the solvent. Different units may be used depending on the specific application and the solute and solvent being used. It’s important to note that the selection of unit will depend on the type of solute and solvent and the purpose of the solution.
- Molarity (M) is the most common unit used to express the concentration of a solution. It is defined as the number of moles of solute per liter of solvent.It is usually denoted by the symbol “M”. For example, a solution of 1 mole of a solute dissolved in 1 liter of solvent would have a molarity of 1 M. Molarity can be used to calculate the number of moles of solute present in a given volume of solution.
- Molality (m) is defined as the number of moles of solute per kilogram of solvent. It is usually denoted by the symbol “m”.
- Mass percentage (mass%) is the ratio of the mass of solute to the mass of the solution multiplied by 100.
- Volume percentage (vol%) is the ratio of the volume of solute to the volume of the solution multiplied by 100.
- Normality (N) is the number of equivalents of solute per liter of solution
- Parts per million (ppm) and parts per billion (ppb) are also commonly used to express the concentration of solutions, particularly in environmental or analytical chemistry. They refer to the ratio of the mass or volume of solute to the mass or volume of the solution, respectively. For example, a solution that contains 1 gram of a solute per 1 million grams of solution would have a concentration of 1 ppm. It’s also used in environmental contexts to express the concentration of pollutants, in food industry to express the presence of certain ingredients or contaminants.
Preparation:
It’s important to note that the preparation of a solution can be affected by various factors such as temperature, pressure, and the chemical nature of the solute and solvent. And also, it’s important to follow proper safety guidelines when handling chemicals.
Preparation of a solution involves dissolving a solute (a substance to be dissolved) in a solvent (the substance that does the dissolving) to create a homogeneous mixture. The process of preparing a solution typically involves the following steps:
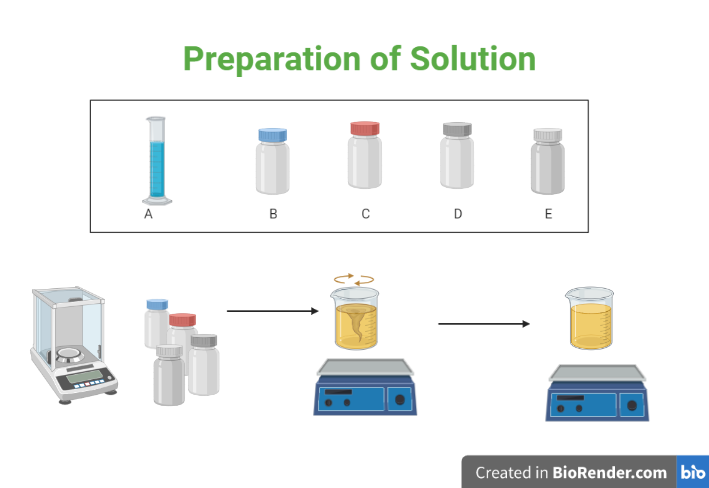
Fig: Preparation of solution
- Selection of solute and solvent: The solute and solvent must be chosen based on the desired concentration of the solution and the chemical compatibility of the solute and solvent.
- Measurement of solute: The amount of solute required for the solution is measured accurately using appropriate laboratory equipment, such as a balance or graduated cylinder.
- Addition of solute to solvent: The solute is added to the solvent in a container, such as a beaker or flask.
- Stirring or heating: The solution is stirred or heated to facilitate the dissolution of the solute. Some solutes dissolve more easily in hot solvents, while others dissolve more easily in cold solvents.
- Filtering: If necessary, the solution can be filtered to remove any undissolved solute or impurities.
- Dilution: If necessary, the solution can be further diluted by adding more solvent to the solution.
- Labelling: The solution should be properly labeled with the name of the solution, the concentration, the date of preparation, and the name of the preparer.
Properties:
Physical
Composition: Solutions are composed of a solute and a solvent, and the concentration of the solute can vary.
Transparency: Most solutions are transparent, meaning that light can pass through them. However, some solutions, such as suspensions and colloids, are opaque or cloudy.
Miscibility: Most solutions are miscible, meaning that they can be mixed together in any proportion without separating. However, some solutions, such as oil and water, are immiscible and will separate if mixed together.
Conductivity: Most solutions are poor conductors of electricity, but electrolyte solutions, which contain ions, can conduct electricity.
Boiling and freezing points: The boiling and freezing points of a solution are usually different from those of the pure solvent. The boiling point of a solution is usually higher, and the freezing point is usually lower, than that of the pure solvent.
Viscosity: Solutions are usually less viscous than pure solvents or solutes, because the solute particles fill in the spaces between the solvent particles, reducing the resistance to flow.
Vapor pressure: The vapor pressure of a solution is usually lower than that of the pure solvent, because some of the solvent molecules are involved in interactions with the solute particles.
Chemical
Solubility: the ability of a substance to dissolve in a solvent.
Concentration: the amount of solute present in a given amount of solvent.
pH: the acidity or basicity of a solution.
Osmotic pressure: the pressure exerted on a semipermeable membrane by a solution.
Conductivity: the ability of a solution to conduct electricity.
Reactivity: the ability of the solution to participate in chemical reactions.
Viscosity: the internal resistance of a fluid to deformation.
Significance:
- In chemistry, solutions are used to study the properties and behavior of different chemical species in different environments.
- In biology and medicine, solutions are used to deliver drugs and other treatments to the body.
- In engineering, solutions are used to solve problems involving heat transfer, fluid flow, and mass transport.
- In food industry, solutions are used to prepare sauces, syrups, jams, jellies, etc.
- In agriculture solutions are used as pesticides and fertilizers.
