Introduction:
It is a method for introducing foreign nucleic acids (e.g., plasmid DNA, cDNA, mRNA, miRNA, siRNA), proteins, and nanoparticles such as beads or dyes into cells usually with the purpose of altering the characteristics of the cells. This procedure can be carried out in a number of ways, including chemical transfection, electroporation, and viral transduction.
Basic Terminology:
- Transfection: The process of introducing foreign genetic material into a cell.
- Transfected cells: Cells that have taken up foreign genetic material during transfection.
- Transfection efficiency: The percentage of cells that have taken up the foreign genetic material.
- Competent cell: This is a cell that has been treated or genetically modified in a way that allows it to take up foreign DNA or plasmid.
- Plasmid: A circular piece of DNA that can replicate independently in a cell. Often used as a vector for transfection to deliver a gene of interest into a cell.
- Vector: A genetic construct used to introduce foreign genetic material into a cell. Vectors can include plasmids, viruses, and other genetic elements.
- Gene expression: The process by which genetic information is used to synthesize a functional product, such as a protein.
- Gene knockdown: The process of reducing the expression of a specific gene through the use of techniques such as RNA interference (RNAi).
- Gene knock-in: The process of introducing a specific gene into a defined location in the genome.
- Gene therapy: A method that uses genetic material to treat or prevent disease.
- Transduction: Transduction is a method of genetic engineering in which DNA is introduced into a cell by a virus. The virus acts as a vector, or carrier, for the DNA. The virus infects the host cell and inserts its genetic material, along with any foreign DNA it is carrying, into the host cell’s genome. Generalized and specialized transduction are the type of transduction that are mainly employed.
Principle:
The objective of transfection is to transport nucleic acids into cells, such as DNA or RNA. Most transfection methods work by creating temporary holes or pores in the cell membrane that allow nucleic acids to enter the cell. Once inside the cell, nucleic acids can be taken up by the cell’s machinery and employed for a variety of functions, including gene expression, knockdown or knockout, and production of protein.
Types:
Importantly, transfection can be divided into two types: stable transfection and transient transfection.
Stable transfection
The process of introducing genetic material into a cell in a way that it becomes a permanent part of the cell’s genome, allowing it to be passed on to daughter cells during cell division. The host genome incorporates foreign DNA, allowing it to be expressed consistently.
Transient transfection
The process of introducing genetic material into a cell that does not become a permanent part of the cell’s genome. Although transported to the nucleus, foreign DNA is not incorporated into the genome. Additionally, foreign mRNA is transported into the cytosol for translation.

Fig: Schematic diagrams of two different transfections
Methods:
Without external stimulus, cells normally do not take up foreign nucleic acids. As a result, an efficient and biocompatible transfection approach that is precisely tailored to the cell type and material to be transmitted is required.
Physical
Physical transfection methods are generally efficient and can achieve high transfection rates, but they can also be technically difficult and necessitate specialized equipment. Furthermore, physical techniques of transfection may cause cell damage or death.
- Electroporation: A high-voltage electrical pulse is applied to cells in the presence of DNA, temporarily creating pores in the cell membrane through which DNA can enter.
- Microinjection: DNA is injected directly into a cell using a fine glass needle. This method is highly efficient, but it is also time-consuming and typically limited to a small number of cells at a time.
- Ballistic transfection: DNA coated nanoparticles is shot into cells using a small device called a gene gun.
- Sonoporation: Ultrasound waves are used to create temporary pores in the cell membrane through which DNA can enter.
- Magnetofection: where DNA is coated with magnetic nanoparticles then magnetic field is applied to cells to pull the particles in the cells.
Chemical
The type of cell, the properties of the genetic material, and the aim of experiment have a role in the decision of which chemical transfection method to choose. Before conducting any experiment, it is highly recommended to evaluate various approaches for optimization because not all chemical transfection techniques are equally effective for all cell types.
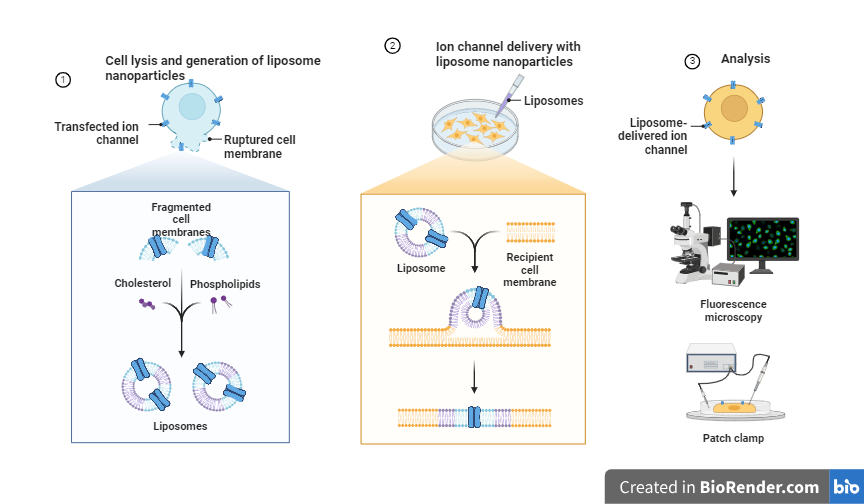
Fig: Liposome mediated transfection
- Lipofection: Lipofection involves the use of liposomes, small spherical vesicles made of phospholipids, to deliver the genetic material into the cell Liposomes are small spherical vesicles made of phospholipids, which can be used as a transfection reagent to deliver foreign genetic material, such as DNA or RNA, into cells. Liposomes can fuse with the cell membrane, releasing their genetic payload into the cell. The transfection efficiency of liposomes can be improved by incorporating a variety of different components into the liposomes, such as cationic lipids, cholesterol, or polyethylene glycol (PEG) to make them more stable, to protect the genetic material from degradation, and to target specific cell types. Lipofection is widely used in transfection and can be used for a variety of cell types including primary cells and stem cells.
- Calcium Phosphate precipitation: This method involves mixing the genetic material with calcium phosphate, which then precipitates and enters the cell through endocytosis. Calcium phosphate precipitation is a simple and widely used method for transfecting primary cells, but it is not as efficient as other methods and can lead to toxicity in some cell types.
- Polyethyleneimine (PEI) transfection: This method involves the use of cationic polymers that can interact with the anionic phospholipids in the cell membrane to create small openings for the foreign genetic material. PEI transfection is considered very efficient, can transfect a variety of cell types but it can lead to cytotoxicity in certain conditions.
- DEAE-dextran transfection: This method uses a DEAE-dextran complex to deliver genetic material into cells. DEAE-dextran transfection is relatively efficient, but it can lead to toxicity in certain cell types.
- Lipid-based transfection reagents: These reagents consist of various cationic lipids and are used in combination with different helper lipids to enable the transfer of DNA or RNA into the cells. they are used in a similar way as lipofection but they may have different properties like increased stability, increased transfection efficiency, or reduced toxicity.
- Others: Many chemical methods have been developed in recent years to improve transfection efficiency, reduce toxicity, and increase versatility, such as polyamidoamine (PAMAM) dendrimers, Polyethylene glycol (PEG, Polyethylenimine (PEI) prodrug conjugates and other polycations or polyanions conjugates.

Fig: Different method of Transfections
Biological
- Viral Transfection: Virus are used as vectors, or carriers, to introduce DNA into cells. This method is highly efficient and can be used to transfect a wide variety of cells, but it can also carry risks such as viral insertional mutagenesis. This method uses a virus, such as a lentivirus, adenovirus, or adeno-associated virus (AAV), as a vector to deliver DNA or RNA into cells.
- Bacterial Transfection: Bacteria are used as vectors to transfer plasmids (circular DNA molecules) containing foreign DNA into cells. This method uses bacteria, like E.coli, to produce large amounts of plasmid DNA and then the plasmid DNA is extracted from the bacteria and used as a vector for transfection.
- Transposon-mediated transfection: Using transposons to promote integration of foreign DNA into the host genome.
- Protoplast transfection: using protoplasts which are cells without cell wall and exposing them to the DNA to allow it to enter the cell.
- CRISPR-mediated transfection: This method uses the CRISPR-Cas system to deliver the Cas enzymes and a guide RNA into cells to enable targeted genome editing.
Workflow:
Preparing the cells: The cells are typically grown in a culture dish or flask until they reach a high enough density for transfection.
Preparing the transfection reagents: This can include creating a solution containing the foreign genetic material (such as plasmid DNA or RNA) and a transfection agent (such as a liposome or polymer).
Transfecting the cells: The transfection reagents are added to the cells in culture. The transfection agent helps the genetic material enter the cells.
Incubating the cells: The cells are incubated for a certain period of time to allow the genetic material to be expressed and for the transfected cells to grow.
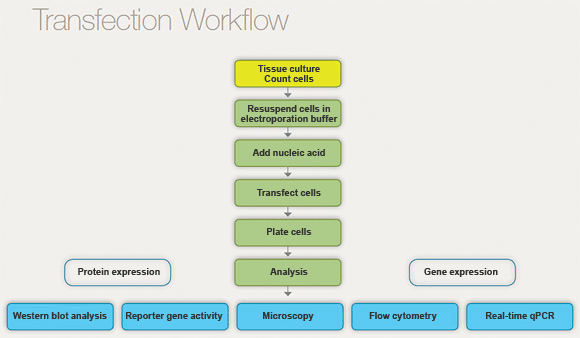
Fig: Workflow of transfection
Analyzing the transfected cells: Depending on the experiment, the transfected cells may be analyzed to confirm that they have taken up the foreign genetic material and that it is being expressed correctly.
Selection or screening of stable transfected cell: After the expression of the target gene, the cells are screened based on the antibiotic resistance or fluorescent protein express, for selecting the stable transfected cells which are used for downstream studies.
Factors Affecting Transfection:
Cell Health
- Cells should be cultivated in the proper medium with all required ingredients.
- Cultures must be free of contamination; if a medium contains chemically unstable ingredients, such as thiamine, fresh medium must be used;
- cells must be maintained in log phase growth;
- cultures must be incubated at 37°C with CO2 supplied at the proper percentage (5-10%) and 100% relative humidity.
Cell Culture
It is recommended to transfect cells when they are between 40 to 80% confluent because too few cells result in poor cell culture growth without cell-to-cell contact, and too many cells cause contact inhibition, which makes cells resistant to cellular uptake of DNA and other macromolecules.
DNA Quality and Quantity
Plasmid DNA must be free of any microbial, chemical, or RNA contamination before being used in transfections. It is strongly recommended to dissolve the ethanol-precipitated DNA in sterile water or TE buffer to a final concentration of 0.2-1 mg/ml. The appropriate amount of DNA to utilize in transfection depends primarily on the type of DNA, transfection reagent/method, target cell line, and number of cells.
Applications:
Transfection is an important and commonly used method in cell biology for studying the activity of numerous genes. This approach can be utilized for gene silencing with RNAi, gene editing with CRISPR/Cas9, and overexpression studies with plasmid DNA, mRNA, or proteins.
Gene expression: Transfection can be used to introduce genes of interest into cells, allowing researchers to study gene function and regulation. This can be useful for understanding the mechanisms of diseases and for developing new therapies.
Modeling diseases: Transfection can be used to introduce mutations or diseased genes into cells, which can be used as a model system to study the mechanisms of a disease.
Protein production: Transfection can be used to introduce genes encoding proteins of interest into cells, which can then be harvested and used for a variety of purposes, such as drug development or biotechnology applications.
Knockdown or Knockout of genes: Transfection can be used to introduce small interfering RNAs (siRNAs) or CRISPR-mediated genome editing tools to suppress or eliminate the expression of a specific gene.
Gene therapy: Transfection can be used to introduce therapeutic genes into cells to correct genetic defects or to treat diseases. This is a promising area of research, with many clinical trials currently underway.
Cancer research: Transfection can be used to introduce oncogenic mutations into cells, which can then be used to study the mechanisms of tumorigenesis.
Stem cell research: Transfection can be used to introduce genetic modifications into stem cells, allowing researchers to study the role of specific genes in the development and differentiation of these cells.
Disadvantages:
Toxicity: Some transfection methods, particularly chemical transfection methods, can be toxic to cells. This can limit the number of cells that can be transfected and can also lead to unintended cell death.
Immune response: Viral transfection methods can elicit an immune response that can be problematic for certain types of cells or organisms, and it may also reduce the longevity of the transfection.
Cost: Some transfection methods, such as viral transfection, can be relatively expensive and require specialized equipment and expertise.
Complexity: Some transfection methods, such as viral transfection, are complex and require specialized expertise and equipment. This can make it difficult to perform in some laboratories.
Host specificity: Some transfection methods may not work well in certain types of cells or organisms, which can limit their utility in certain types of studies.
Limited genome editing capabilities: Not all the transfection methods allow editing of the genome, and those that allow it may have limitations in the specificity or the efficiency of the editing.
References:
- Kim, T.K. and Eberwine, J.H., 2010. Mammalian cell transfection: the present and the future. Analytical and bioanalytical chemistry, 397(8), pp.3173-3178.
- https://www.bio-rad.com/transfection
- Schenborn, E.T. and Goiffon, V., 2000. DEAE-dextran transfection of mammalian cultured cells. Transcription Factor Protocols, pp.147-153.
- Fus-Kujawa, A., Prus, P., Bajdak-Rusinek, K., Teper, P., Gawron, K., Kowalczuk, A. and Sieron, A.L., 2021. An overview of methods and tools for transfection of eukaryotic cells in vitro. Frontiers in Bioengineering and Biotechnology, p.634.
