Introduction:
- Autophagy is a cellular process that involves the degradation and recycling of cellular components. It is a normal and regulated process that occurs in cells in response to stress or nutrient deprivation, and it plays a crucial role in maintaining cellular homeostasis.
- The word “autophagy” is derived from the Greek words “auto-” meaning “self” and “phagein” meaning “to eat.” It was coined by Belgian biochemist Christian de Duve in the 1960s, who was awarded the Nobel Prize in Medicine in 1974 for his work on the discovery and characterization of lysosomes and autophagy.
- It is a process through which a cell breaks down and destroys old, damaged, or aberrant proteins and other components in its cytoplasm. The breakdown products are then recycled for crucial cell functions, particularly during times of stress or starvation. Autophagy also aids in the destruction of bacteria and viruses that cause infection and may protect normal cells from mutating into cancer cells.
- Once cancer has formed, autophagy may protect the cancer cells by supplying extra nutrients to them or by preventing anticancer medications or other substances from destroying them.
- Autophagy may also influence the body’s immunological response to viruses, bacteria, and cancer cells.
Mechanism:
Initiation: Autophagy is initiated by the activation of autophagy-related genes, which leads to the formation of a double-membraned vesicle called the autophagosome.
Nucleation: The autophagosome is formed through a process called nucleation, which involves the assembly of a protein complex called the phagophore assembly site (PAS). The PAS consists of several proteins, including the protein ATG13 and the serine/threonine kinase ULK1. Autophagy-related proteins (ATGs) proteins, a group of proteins involved in autophagy, play a crucial role in the initiation and regulation of this process. Dysregulation of ATG proteins has been linked to a number of diseases, including cancer, neurodegeneration, and metabolic disorders.
Elongation: The autophagosome then grows in size by incorporating more material from the surrounding cytoplasm. This process is facilitated by proteins called ATG proteins, which are involved in the biogenesis of the autophagosome.
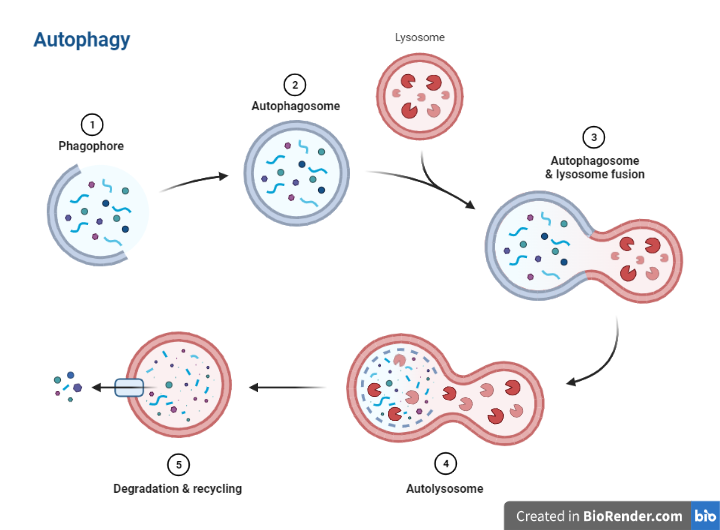
Fig: Process of autophagy
Fusion: The autophagosome then fuses with a lysosome, a vesicle containing hydrolytic enzymes, to form an autolysosome.
Maturation: Once the autophagosome has reached a sufficient size, it becomes more spherical in shape and begins to mature. During this process, the autophagosome fuses with lysosomes, which contain hydrolytic enzymes that can break down the material inside the autophagosome.
Degradation: The material inside the autophagosome is degraded by the hydrolytic enzymes in the lysosome, and the resulting products are recycled for use by the cell.
Termination: Autophagy is terminated when the autophagosome is fully degraded and the autophagic process is no longer needed.
Types:
Autophagy is a cellular degradation and recycling mechanism that is observed in all eukaryotes. Microautophagy, macroautophagy, and chaperone-mediated autophagy are the three main kinds of autophagy in mammalian cells (CMA). While each is morphologically distinct, all three culminate in the transport of cargo to the lysosome for destruction and recycling.
Macroautophagy
This phase involves the activation of autophagy-related proteins (ATGs) and the formation of the phagophore, which is an empty double-membrane structure that will eventually become the autophagosome.
In this case, de novo synthesis of double-membrane vesicles—autophagosomes—is used to sequester cargo which encloses cellular material to be degraded and recycled. and subsequently transport it to the lysosome. The autophagosome then fuses with a lysosome, which contains hydrolytic enzymes that can break down the enclosed material.
Macroautophagy occurs naturally at a low level and can be activated further under stress situations, such as nutrient or energy deprivation, to digest cytoplasmic material into metabolites that can be utilized in biosynthetic activities or energy production, allowing cell survival.
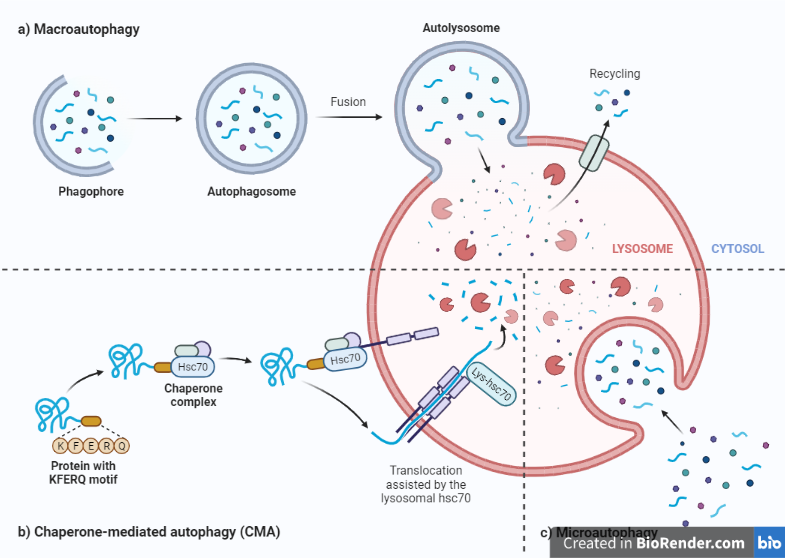
Fig: Types of autophagy
Microautophagy
It is a type of autophagy that involves the direct engulfment of cytoplasmic material by the lysosome. It is a more selective process than macroautophagy, as it involves the direct recognition and engulfment of specific cellular material by the lysosome.
During microautophagy, the lysosome forms a small invagination on its membrane, which then extends and envelops the target material. The lysosome then fuses with the invagination, enclosing the material within its own lumen. The hydrolytic enzymes within the lysosome can then break down the enclosed material, and the products of this degradation are recycled for use in other cellular processes.
Chaperone-mediated autophagy
This is a specialized form of protein degradation that involves the recognition of specific proteins by chaperone molecules, which then guide them to the lysosome for degradation. CMA is a highly selective process, as it targets specific proteins for degradation based on the presence of a specific amino acid sequence called the KFERQ motif.
During CMA, the chaperone molecule called Hsc70 recognizes the KFERQ motif on the target protein and binds to it. The complex then interacts with the lysosomal membrane protein LAMP-2A, which allows the target protein to be transported into the lysosome for degradation.
CMA is thought to play a role in the degradation of damaged or misfolded proteins, as well as in the turnover of specific proteins that are important for cellular homeostasis.
Regulations:
There are several signaling pathways and proteins that regulate the process of autophagy, including:
Target of rapamycin (TOR) pathway: The TOR pathway is a conserved signaling pathway that senses the availability of nutrients and energy in the cell and regulates cell growth and proliferation accordingly. When nutrients and energy are abundant, the TOR pathway is active and promotes cell growth and proliferation. When nutrients and energy are scarce, the TOR pathway is inhibited, which can lead to the activation of autophagy.
AMP-activated protein kinase (AMPK) pathway: The AMPK pathway is activated in response to cellular stress, such as energy deprivation or oxidative stress. Activation of the AMPK pathway can lead to the inhibition of the TOR pathway and the activation of autophagy.
Protein kinase B (Akt) pathway: The Akt pathway is a signaling pathway that promotes cell survival and growth. Inhibition of the Akt pathway has been shown to lead to the activation of autophagy.
Nitrogen-dependent regulation: Autophagy is a process by which cells recycle their own components, including damaged or unnecessary organelles and proteins. Nitrogen plays a role in the regulation of autophagy in cells. When nitrogen levels are low, autophagy is induced as a way for the cell to conserve nitrogen. When nitrogen levels are high, autophagy is inhibited. Nitrogen is an important component of amino acids, which are the building blocks of proteins. When nitrogen levels are low, the cell may be unable to synthesize new proteins and may instead recycle existing proteins through autophagy. Nitrogen-dependent regulation of autophagy is thought to play a role in the response of cells to changes in their environment and in the maintenance of homeostasis.
Energy/glucose-dependent regulation: Autophagy is also regulated by the availability of energy and glucose in cells. When energy levels are low, autophagy is induced as a way for the cell to generate energy by breaking down and recycling its own components. This process is known as “starvation-induced autophagy.” Conversely, when energy levels are high, autophagy is inhibited.
Glucose also plays a role in the regulation of autophagy. When glucose levels are low, autophagy is induced as a way for the cell to generate energy by breaking down and recycling its own components. This process is known as “glucose deprivation-induced autophagy.” When glucose levels are high, autophagy is inhibited.
Energy and glucose-dependent regulation of autophagy is thought to play a role in the response of cells to changes in their environment and in the maintenance of homeostasis.
Autophagy-related proteins (ATGs): ATGs are a group of proteins that are involved in the regulation of autophagy. They play a key role in the initiation, elongation, and maturation phases of autophagy and include proteins such as ULK1, ATG5, and ATG12.
Physiological roles of autophagy:
Homeostasis: Autophagy helps to maintain homeostasis by removing damaged or unnecessary organelles and proteins from cells.
Development and differentiation: Autophagy is involved in the development and differentiation of cells during embryonic development and tissue repair.
Immunity: Autophagy helps to defend against infection by degrading and eliminating invading pathogens within cells. It is also involved in the development and function of immune cells.
Aging: Autophagy is thought to play a role in the aging process and in the development of age-related diseases.
Energy metabolism: Autophagy is involved in the regulation of energy metabolism by providing a source of energy for the cell during times of stress or starvation.
Neurodegenerative disorders: Autophagy is thought to be involved in the pathogenesis of neurodegenerative disorders, such as Parkinson’s disease and Alzheimer’s disease.
Cancer: Autophagy can have both pro- and anti-tumorigenic effects, depending on the context. In some cases, autophagy may help to suppress the development of cancer by eliminating damaged or abnormal cells, while in other cases it may promote the survival and growth of cancer cells.
Autophagy and disease:
Autophagy has been linked to the development and progression of a number of diseases. Dysregulation of autophagy has been implicated in the pathogenesis of a variety of disorders, including:
Cancer: Autophagy can have both pro- and anti-tumorigenic effects, depending on the context. In some cases, autophagy may help to suppress the development of cancer by eliminating damaged or abnormal cells, while in other cases it may promote the survival and growth of cancer cells.
Neurodegenerative disorders: Autophagy is thought to be involved in the clearance of abnormal proteins that accumulate in neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease. Dysregulation of autophagy may contribute to the development and progression of these disorders.
Diabetes: Autophagy has been linked to the development and progression of diabetes, both type 1 and type 2. Dysregulation of autophagy may contribute to the development of insulin resistance and the inability of cells to properly utilize glucose.
Cardiovascular disease: Autophagy has been linked to the development of cardiovascular disease, including atherosclerosis and heart failure. Dysregulation of autophagy may contribute to the development of these disorders.
Aging: Autophagy is thought to play a role in the aging process and in the development of age-related diseases. Dysregulation of autophagy may contribute to the development of these disorders.
Infections: Autophagy is involved in the defense against infection by degrading and eliminating invading pathogens within cells. Dysregulation of autophagy may contribute to the development and progression of infections.
References:
- Benbrook DM, and Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp. Oncol. 2012; 34(3):286-97. [PMID: 23070014]
- Levine B, Kroemer G: Autophagy in the pathogenesis of disease. Cell. 2008, 132: 27-42. 10.1016/j.cell.2007.12.018.
- Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014 Jan 20;20(3):460-73.
- Yin, Z., Pascual, C. and Klionsky, D.J., 2016. Autophagy: machinery and regulation. Microbial cell, 3(12), p.588.
